Polemoniaceae
Phlox Family
Leigh Johnson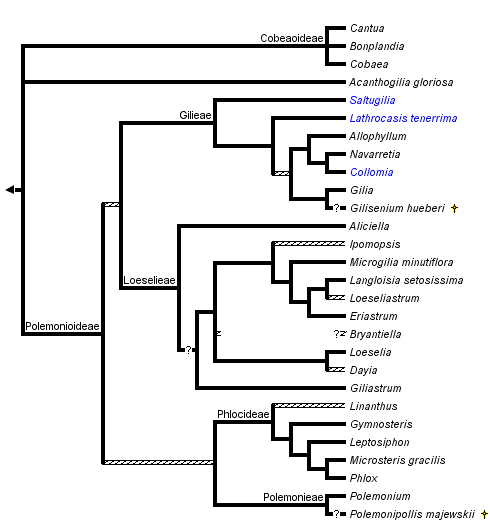


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Polemoniaceae, the phlox family, includes about 400 species of herbs, shrubs, small trees, and vines. Some members of this family are common desert, montane, or woodland wildflowers that form spectacular displays during their brief blooming periods. Scarlet gilia (Ipomopsis aggregata), with showy, bright red flowers, is one of the most obvious and frequently collected summer wildflowers in its range. Few species in this family are economically important, although some are commonly grown in home gardens, and others are found in herbal dietary supplements. In the plant sciences, the phlox family is an important model for investigating pollination biology and evolutionary patterns (e.g. Galen, 2000; Mayfield et al., 2001; Campbell et al., 2003; Lendvai and Levin, 2003). Various species are adapted for pollination by bats, hummingbirds, bees, both short- and long-tongued flies, beetles, butterflies, or moths (Grant and Grant, 1965). Important insights into plant speciation, especially among annual plants, have been gained from studies of species in this family (Grant, 1981). Many interesting patterns of variation that can further enlighten our understanding of diversification modes and mechanisms remain to be addressed.
Characteristics
No single morphological feature uniquely identifies a member of the Phlox family. However, a combination of flower traits is useful for distinguishing Polemoniaceae from other families of flowering plants. These identifying features are: five sepals, five fused petals, five stamens that alternate with the corolla lobes, and an ovary of three fused carpels. Stamens may be superficially connected to the corolla in some lineages, but are fused completely into the corolla tube in most species. The three-parted ovary, in combination with the other floral features, is key. Many asterid families have a similar arrangement of petals and stamens, but in these families the primitive number of carpels is two. Allied families with three carpels, such as Fouquieriaceae and Diapensiaceae, differ in stamen features and other particulars (Johnson et al., 1999). Only a few scattered species of Polemoniaceae in Linanthus and Navarretia depart from this formula by being four- or six-merous, or having just two carpels. The phylogenetic placement of these exceptional taxa within the family indicates that the common floral pattern is primitive in this family and deviations are derived.


Typical Polemoniaceae flowers. A. Polemonium occidentale, fresh flowers. The three stigmatic lobes, indicating that three carpels form the ovary, are apparent in the topmost flower. Inset is a close up of the ovary, resting on a thin nectary disk. B. Saltugilia latimeri, pressed flower rehydrated in Pohl's solution and dissected. Inset is a close-up of the three stigmatic lobes with long papillae and several pollen grains attached. From L-R : open corolla showing five stamens attached to corolla tube, calyx tube with five segments joined by a thin membrane, and ovary with style and three stigmatic lobes. Images copyright © 2003 L. Johnson.
Other common features of Polemoniaceae include a nectary disk at the base of the ovary, typically the mature fruit is a loculicidal capsule (septicidal in Cobaea), and the petals lobes overlap each other on one edge in bud (convolute aestivation; but aestivation is imbricate in some Cantua). Foliage leaves are arranged either opposite or alternate each other along the stem (or lacking in Gymnosteris), and vary from entire to dissected, compound, and even compound with the terminal leaflet modified into a tendril (Cobaea). In life history, many Polemoniaceae are annual plants that germinate, flower, and die within a twelve-month period. Many others, however, are perennial and polycarpic (persist more than two years and flower multiple times over their lives) and a few are biennial (two-year lifespan). Habit varies from delicate herbs to woody shrubs to rare small trees (Cantua) and woody vines (Cobaea).
Geographic Distribution
The majority of the phlox family grows natively in western North America. The diversity of species and genera is particularly high in California, USA, where 17 genera and over 170 species occur. Significant diversity also exists elsewhere in the world. A principle lineage consisting of Cantua, Cobaea, and Bonplandia is distributed natively only in Mexico, central America, and northwestern South America. In Eastern North America, the genus Phlox is unusually diverse compared to the rest of the family. Other than members of Phlox, only a few species occur natively east of the Mississippi River in the United States, such as Ipomopsis rubra, Collomia linearis, and a few Polemonium. A few Phlox and Polemonium species are also found natively in Eurasia. Central and South America, particularly the western regions of South America, are also home to some members of the phlox family. A small number of species from several genera that are most abundant in North America, such as Collomia, Gilia, Navarretia, and Leptosiphon, are found only in western South America, indicating multiple instances of long distance dispersal (Grant 1959; Morrell et al. 2000). Occasional species, such as skunkweed (Navarretia squarrosa) and Collomia grandiflora, have become weedy in parts of Europe and Australia, and some cultivated species, such as members of Cobaea, have become naturalized outside their native range (Grant, 1959; Hussey et al., 1997; Pysek et al., 2002; Anon., 2003).
Popular Culture and Ethnobotany
The beauty of Polemoniaceae has brought their flowers into popular culture in different regions of the world. Kantuta (Cantua buxiolia) is the national flower of Bolivia and Peru. Known also as "magic flower" and "sacred flower of the Incas", the colors of the flower match the red, yellow, and green stripes on the Bolivian flag. A latin dance band from New Zealand, with three albums as of 2008, is named Kantuta after this flower. In Sonthofen, Germany, "Phlox: food and motion" is a café and vinothek that uses artwork of phlox flowers as a motif in their logo and interior decoration. The Sanford Winery, located in the Santa Ynez Valley of California, USA, has used various Polemoniaceae on their trademark wildflower labels that mark each vintage and year.


Cantua buxifolia in La Paz, Bolivia. Image copyright © 1998 Lynn Bohs.
Historically, many Polemoniaceae species have been exploited by native American cultures for a wide range of uses. Over 40 species from eight genera were used by native tribes in North America (Moerman, 1998), with uses ranging from utilitarian (leaves to cover berry baskets), food (from seeds), hygiene (face and hair washing) to general (fever) or specific (scorpion stings) medicinal treatments. Brand (1907) indicates Peruvian Indians made use of a soapy solution derived from Cantua pyrifolia leaves for laundry. Espinolla, one of several common names for Loeselia coccinea, a popular herb in Mexico, is becoming more common in the herbal industry worldwide, and is reputedly useful for treating respiratory diseases, stomach inflammation, and postchildbirth fever.
Horticulture
All but the smallest-flowered genera have species suitable for cultivation in a variety of settings (rock and container gardens, formal borders, naturalized wildflower areas; Huxley et al., 1992). However, only Phlox (moss pinks, garden phlox, wild sweet williams) and Polemonium (Jacob's ladder, skypilot) are commonly available for use in home landscapes. Seeds of bird's eye-gilia (Gilia tricolor), cathedral bells (Cobaea scandens, also known as cup-and-saucer vine), and a few other species can also be found from regional sources or more specialized retailers.
(Left) Phlox subulata 'Candystripe'. Image copyright © 2003 Leigh Johnson. (Right) Polemonium caeruleum 'Brise d' Anjou'. Variegated Jacob's Ladder. Image copyright © 2003 Paula Squitiere.
Conservation
As of 2008, eight species (10 taxa) of Polemoniaceae from five genera within the United States are listed on the U.S. Federal Register as either threatened (two species) or endangered (six species). Several additional species are narrowly distributed or restricted to specialized habitats, and many of these are listed as threatened, endangered, or as species of concern by individual states. Additional species of conservation concern are known from areas outside the United States, such as Baja California, but formal conservation status is uncertain for most of these.
Fossil Record
Few species of Polemoniaceae inhabit environments where burial, sedimentation, and ultimately fossilization are likely to occur. Consequently, the fossil record of Polemoniaceae is poor. Lott et al. (1998) review the fossil history of this family, which consists primarily of pollen grains: from the middle Eocene in Spain, from the Pliocene in Europe (attributed to a fossil-genus, Polemoniopollis), and Gilia type 1-like pollen (Stuchlik, 1967) from Miocene deposits in Northern California. The only megafossil currently contributed to Polemoniaceae, Gilisenium hueberi, is a nicely preserved compression-impression fossil from mid-eocene shale deposits taken from the Green River Formation in Utah (USA; Lott et al., 1998). This fossil of a taprooted, herbaceous plant is very similar to extant Gilia species and provides an important calibration point for estimating the timing of the origin of the phlox family and various groups within this family.


Gilisenium hueberi, Type specimen. This is the sole macrofossil of Polemoniaceae. Image copyright © 2003, courtesy of the Department of Paleobiology, National Museum of Natural History, Smithsonian Institution.
Discussion of Phylogenetic Relationships
The monophyly of Polemoniaceae is strongly supported by cladistic analyses of DNA sequence data. The branch uniting Polemoniaceae shows a high level of character support in chloroplast matK (Johnson et al., 1996), chloroplast ndhF (Prather et al., 2000) and nuclear rDNA 18S (Johnson et al., 1999) sequence analyses. Although a cladistic analysis to assess the monophyly of the phlox family has not been conducted with morphology, monophyly can be inferred by a unique combination of morphological features that center on floral and reproductive characters (see discussion above and in Johnson et al., 1999). Historically, there has been little questioning of the limits of this family although Cobaea has sometimes been segregated into its own family, Cobaeaceae, based on its vining habit, septicidal capsules, and other distinctive morphological features (Hutchinson, 1959). Cladistic analyses, all currently DNA-sequence based, strongly indicate a derived position for Cobaea within Polemoniaceae, rather than a sister relationship to all other genera (see also Prather, 1999).
Phylogenetic reconstruction within the phlox family is a work in progress. Early cladistic analyses of nuclear rDNA ITS sequences (Porter, 1996) and chloroplast matK (Johnson et al., 1996) and ndhF (Prather et al., 2000) sequences generally agree in recognizing six major groups of Polemoniaceae that have been recognized taxonomically at either the tribal or subfamily level (Porter and Johnson, 2000). Separate and combined analyses of several chloroplast DNA regions and the nuclear rDNA ITS regions for 84 species representing the 26 genera recognized by Porter and Johnson (2000) further support these higher-level groups (Johnson et al., 2008). Support for relationships within and among these lineages varies, and additional work is needed to resolve the relationships among these major groups. Furthermore, while the clades ranked as tribes and subfamilies by Porter and Johnson (2000), particularly in subfamily Polemonioideae (all genera except Acanthogilia, Bonplandia, Cobaea, and Cantua), are supported to greater or lesser degrees by molecular data, well-defined morphological synapomorphies exclusive to these groups are lacking in many cases. Taxon descriptions for super-generic groups include ranges of morphological variation that are rarely unique to a single, higher-level taxon. Consequently, meaningful taxonomic diagnoses are difficult to construct, and terminal taxa for the phylogenetic hypothesis presented above are genera (or species when genera are monotypic) rather than tribes or subfamilies. In general, polytomies in this phylogenetic hypothesis indicate relationships that are weakly supported in all data sets.
Prather et al. (2000) summarize disagreement among molecular studies in the placement of Acanthogilia gloriosa. Based on ndhF sequences, these authors favor a sister relationship between Acanthogilia and Bonplandia+Cantua+Cobaea. However, this relationship is only weakly supported, and is in disagreement with other published analyses that, similarly, disagree with each other. Combined analyses of five chloroplast regions and these regions plus nrDNA ITS sequences do not resolve the position of this species (Johnson et al., 2008). Acanthogilia represents an important lineage in the early diversification of Polemoniaceae and is either sister to all other Polemoniaceae, to just Bonplandia+Cantua+Cobaea, or to all other Polemoniaceae except these three genera.
Bonplandia+Cantua+Cobaea form a monophyletic group. In cpDNA studies, Bonplandia is sister to Cobaea, and these two genera combined are sister to Cantua; nrITS sequences place Cobaea and Cantua as sisters. Neither alternative is strongly supported.
The remaining genera of Polemoniaceae, are well supported as a monophyletic group composed of four principal groups: Polemonieae (Polemonium and Polemoniopollis); Gilieae (Saltugilia, Gilia, Collomia, Navarretia, Allophyllum and Lathrocasis); Phlocideae (Linanthus, Gymnosteris, Leptosiphon, Phlox, and Microsteris); and Loeselieae (Aliciella, Dayia, Loeselia, Giliastrum, Bryantiella, Ipomopsis, Microgilia, Eriastrum, Langloisia, and Loeseliastrum). Relationships among these groups are not strongly supported. Polemonieae is consistently resolved as sister to Phlocideae in both nrITS and a matrix of five cpDNA regions (Johnson et al., 2008). This same study resolves Loeselieae and Gilieae as sister to each other. Bayesian posterior probabilities for these relationships are high, but parsimony bootstrap values are week.
Polemonieae contains the single extant genus Polemonium. Fossil Polemonium-like pollen grains from Pliocene deposits in Europe have been described as Polemonipollis majewskii (Krutzsch, 1966; Muller, 1981). This taxon is inferred from the scanty morphological evidence available to be allied with Polemonium.
Within Gilieae, Saltugilia is strongly supported as sister to the remainder of the tribe, and Lathrocasis may be sister to the remaing genera. DNA sequence data suggest Lathrocasis is sister to Gilia, Collomia+Navarretia+Allophyllum, or both of these groups combined (Johnson and Weese, 2000; Johnson et al., 2008). Though the seed coat of Lathrocasis is unique, Gilia shares a seed coat morphology with Collomia, Allophyllum, and Navarretia (Johnson et al., 2004). Gilisenium, a fossil genus, has an inferred relationship near Gilia based on readily apparent morphological similarities (Lott et al. 1998), but has not been directly included in cladistic analyses. It could also belong in Loeselieae. Allophyllum, Collomia, and Navarretia form a very well support clade.
Within Phlocideae, relationships among genera are largely resolved with molecular data. Linanthus shows some indications, mostly surrounding the taxon Linanthus inyoensis, of being paraphyletic with respect to the rest of the tribe, and support for monophyly of Linanthus is the weakest among genera in this tribe (Bell and Patterson, 2000). Additional morphological and molecular analyses are needed to resolve relationships with robustness at the base of this tribe. Microsteris, which has been treated within Phlox by various authors, remains supported as sister to Phlox in published analyses (Ferguson and Jansen, 2002).
Among major lineages, tribe Loeselieae has the weakest support for monophyly in the family, but this clade is consistently recovered in molecular analyses. The radiation of lineages appears to have occurred rapidly relative to rates of molecular evolution in sampled genes, giving little resolution among many of the genera in this clade. Although several genera are well-supported as monophyletic, such as Aliciella, Giliastrum, and Eriastrum, others are questionable at present. Additional work by J. M. Porter (Rancho Santa Ana Botanic Garden, unpublished), indicates Bryantiella may not be monophyletic. These same data show that Ipomopsis as presently circumscribed also contains lineages that lack most recent common ancestry with the bulk of this genus. Further morphological and molecular work focused on Loeseliastrum is also warranted to resolve the affinities and evolutionary history of L. depressum, a species recently transferred to this genus but with discordant affinities in ITS versus chloroplast sequence data.
Classification of Polemoniaceae
A phylogeny-based classification that places Polemoniaceae genera into tribes and subfamilies has been proposed that recognizes well-supported nodes from a growing body of comparative DNA sequence studies and non-cladistic consideration of morphology (Porter and Johnson, 2000). This classification is subject to revision as additional data that bear on phylogenetic branching patterns are brought forth, with an emphasis on recognizing monophyletic groups that include all descendents of a common ancestor. Driven by this philosophy, some changes to the classification of Porter and Johnson (2000) will be forthcoming as relationships in groups of questionable monophyly are addressed, and polytomies are resolved. For example, based on resolution of Acanthogilia as sister to Cobaea, Bonplandia, and Cantua, Prather et al (2000) suggested that Acanthogilia be included in subfamily Cobeaoideae. An alternative classification that reflects this same phylogenetic branching pattern could also be proposed by maintaining Cobeaoideae as circumscribed phylogenetically by Porter and Johnson (2000), and erecting a new, super subfamilial taxon that circumscribes this subfamily + Acanthogilia.
An alternative classification for Polemoniaceae based on interpretations of overall similarity has also been proposed, and revised, in recent years (Grant 1998a,b, Grant and Day 1998, Grant 2003b). This classification adheres to a broader definition of monophyly that allows paraphyletic groups, and considers phylogenetic patterns of secondary importance (Grant 2003a).
References
Anon. 2003. APNI: Australian Plant Names Index. http://www.anbg.gov.au/cpbr/databases/apni.html.
Bell, C. D. and R. W. Patterson. 2000. Molecular phylogeny and biogeography of Linanthus (Polemoniaceae). Am. J. Bot. 87:1857-1870.
Brand, A. 1907. Polemoniaceae. Pages 1-203 in A Engler, ed. Das Pflanzenreich IV(250). Engelmann, Leipzig.
Campbell, D.R., R. Alarcon, and C. A. Wu. 2003. Reproductive isolation and hybrid pollen disadvantage in Ipomopsis. J. Evolution. Biol. 16:536-540.
Carlquist, S., V. M. Eckhart, and D. C. Michener. 1984. Wood anatomy of Polemoniaceae. Aliso 10:547-572.
Dawson, M. L. 1936. The floral morphology of the Polemoniaceae. Am. J. Bot. 23: 501-511.
Ferguson, C. J. and R. K. Jansen. 2002. A chloroplast DNA phylogeny of eastern Phlox (Polemoniaceae): implications of congruence and incongruence with the ITS phylogeny. Am. J. Bot. 89:1324-1335.
Galen, C. 2000. High and dry: Drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). Am. Nat. 156:72-83.
Grant, V. 1981. Plant speciation. Columbia University Press, New York, NY.
Grant, V. 2002. Frequency of spontaneous amphiploids in Gilia (Polemoniaceae) hybrids. Am. J. Bot. 89:1197-1202.
Grant, V. 1959. Natural history of the phlox family. Martinus Nijhoff, The Hague, Netherlands.
Grant, V. 1998a Primary classification and phylogeny of the Polemoniaceae, with comments on molecular cladistics. Am. J. Bot. 85:741-752.
Grant, V. 1998b [1999] Classification of the genus Gilia (Polemoniaceae). Phytologia 84:69-86.
Grant, V. 2003a. Incongruence between cladistic and taxonomic systems. American Journal of Botany 90: 1263-1270.
Grant, V. 2003b. Taxonomy of the Polemoniaceae: the subfamilies and tribes. Sida 20: 1371-1385.
Grant, V., and A. G. Day. 1998 [1999]. Transfer of some species of Gilia to Allophyllum and Tintinabulum, and the effects of the transfer on the generic definition of Gilia (Polemoniaceae). Phytologia 84:368-382.
Grant, V. and K. A. Grant. 1965. Flower pollination in the Phlox family. Columbia University Press, New York, NY.
Harborne, J. B., and D. M. Smith. 1978. Correlations between anthocyanin chemistry and pollination ecology in the Polemoniaceae. Biochem. Syst. Ecol. 6:127-130.
Hussey, B. M. J., G. J. Keighery, R. D. Cousens, J. Dodd, and S. G. Lloyd. 1997. Western weeds: a guide to the weeds of Western Australia. The Plant Protection Society of Western Australia , Victoria Park.
Hutchinson, J. 1959. The families of flowering plants, 2nd edition. Clarendon Press, Oxford.
Huxley, A., M. Griffiths, and M. Levy (eds.) 1992. The New Royal Horticultural Society Dictionary of Gardening, Vol. 1. Macmillan Press Ltd., London.
Johnson, L. A., L. M. Chan, T. L. Weese, L. D. Busby, and S. McMurry. 2008. Nuclear and cpDNA sequences combined provide strong inference of higher phylogenetic relationships in the phlox family (Polemoniaceae). Molecular Phylogenetics and Evolution 48:997-1012.
Johnson, L. A., K. H. Holt, and J. M. Porter. 2004. Seed surface sculpturing and its systematic significance in Gilia (Polemoniaceae) and segregate genera. International Journal of Plant Sciences 165:153-172.
Johnson, L. A. and T. L. Weese. 2000. Geographic distribution, morphological and molecular characterization, and relationships of Lathrocasis tenerrima (Polemoniaceae). W. N. Am. Nat. 60:355-373.
Johnson, L. A., D. E. Soltis, and P. S. Soltis. 1999. Phylogenetic affinities of Polemoniaceae inferred from nuclear ribosomal 18S DNA sequences. Plant Syst. Evol. 214:65-89.
Johnson, L. A., J. L. Schultz, D. E. Soltis, and P. S. Soltis. 1996. Monophyly and generic relationships of Polemoniaceae based on matK sequences. Am. J. Bot. 83:1207-1224.
Kapil, R. N., P. N. Rustagi, and R. Venkataraman. 1968. A contribution to the embryology of Polemoniaceae. Phytomorphology 18:403-411.
Krutzsch, W. 1966. Zur Kenntnis der präquartären perporaten Pollenformen. Geologie 15:16-71.
Lendvai, G., and D. A. Levin. 2003. Rapid response to artificial selection on flower size in Phlox. Heredity 90:336-342.
Lott, T.A., S. R. Manchester, D. L. Dilcher. 1998. A unique and complete polemoniaceous plant from the middle Eocene of Utah, USA. Rev. Palaeobot. Palyn. 104:39-49.
Mayfield, M. M., N. M. Waser, and M.V. Price. 2001. Exploring the 'most effective pollinator principle' with complex flowers: Bumblebees and Ipomopsis aggregata. Ann. Bot-London 88:591-596.
Moerman, D. E. 1998. Native American Ethnobotany. Timber Press, Portland, OR.
Morrell, P. L., J. M. Porter, and E. A. Friar. 2000. Intercontinental dispersal: the origin of the widespread South American plant species Gilia laciniata ( Polemoniaceae ) from a rare California and Oregon coastal endemic. Plant Syst. Evol. 224:13-32.
Muller, J. 1981. Fossil pollen records of extant angiosperms. Bot. Rev. 47:1-142.
Plitmann, U. and D. Levin. 1983. Pollen-pistil relationships in the Polemoniaceae. Evol. 37:957-967.
Plitmann, U. and D. Levin. 1990. Breeding systems in the Polemoniaceae. Pl. Syst. Evol. 170:205-214.
Porter, J. M. 1996 [1997]. Phylogeny of Polemoniaceae based on nuclear ribosomal internal transcribed spacer DNA sequences. Aliso 15:57-77.
Porter, J. M. and L. A. Johnson. 1998. Phylogenetic relationships of Polemoniaceae: inferences from mitochondrial nad1 intron sequences. Aliso 17:157-188.
Porter, J. M., and L. A. Johnson. 2000. A phylogenetic classification of Polemoniaceae. Aliso 19:55-91.
Prather, L. A. 1999. Systematics of Cobaea (Polemoniaceae). Syst. Bot. Monogr. 57:1-81.
Prather, L. A., C. J. Ferguson, and R. K. Jansen. 2000. Polemoniaceae phylogeny and classification: implications of sequence data from the chloroplast gene ndhF. Am. J. Bot. 87:1300-1308.
Pysek, P., Sadlo J. and B. Mandak. 2002. Catalogue of alien plants of the Czech Republic. Preslia, Praha 74:97-186.
Smith, D. M, C. W. Glennie, and J. B. Harborne. 1982. Flavonoid patterns in Leptodactylon and Linanthus. Biochem. Syst. Ecol. 10:37-42.
Smith, D. M, C. W. Glennie, J. B. Harborne and C. A. Williams. 1977. Flavonoid diversification in the Polemoniaceae. Biochem. Syst. Ecol. 5:107-115
Steele, K. P., and R. Vilgalys. 1994. Phylogenetic analysis of Polemoniaceae using nucleotide sequences of the plastid gene matK. Syst. Bot. 19:126-142.
Stuchlik, L. 1967a. Pollen morphology in the Polemoniaceae. Grana Palynol. 7:146-240.
Stuchlik, L. 1967b. Pollen morphology and taxonomy of the family Polemoniaceae. Rev. Palaeobot. Palynol. 4:325-333.
Taylor, T. N., and D. A. Levin. 1975. Pollen morphology of Polemoniaceae in relation to systematics and pollination systems: scanning electron microscopy. Grana 15:91-112.
Wherry, E. T. 1940. A provisional key to the Polemoniaceae. Bartonia 20:14-17.
Information on the Internet
Ethnobotany (not restricted to just Polemoniaceae)- Native American Ethnobotany
- Ethnobotany of the INEEL
- Luiseño Ethnobotany
- Compendium of natural and alternative medicine (in Spanish)
Title Illustrations

| Scientific Name | Phlox amoena |
|---|---|
| Location | USA: North Carolina Botanic Garden, Cultivated. |
| Specimen Condition | Live Specimen |
| Sex | Perfect flowers |
| Body Part | Inflorescence |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2003

|
| Scientific Name | Ipomopsis aggregata |
|---|---|
| Location | USA: Utah Co., Utah |
| Specimen Condition | Live Specimen |
| Sex | Perfect flowers |
| Body Part | Inflorescence |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2003

|
| Scientific Name | Gilia stellata |
|---|---|
| Location | USA: Washington Co., Utah |
| Specimen Condition | Live Specimen |
| Sex | Perfect flowers |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2003

|
| Scientific Name | Polemonium foliosissimum |
|---|---|
| Location | USA: Juab Co., Utah |
| Specimen Condition | Live Specimen |
| Sex | Perfect flowers |
| Body Part | upper 2/3 of plant |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 2003

|
About This Page
Work on the Polemoniaceae Tree of Life pages was supported by NSF grant DEB-0344873 to Leigh A. Johnson. Special thanks to P. Squitiere, L. Bohs, and the Smithsonian Institution for permission to post certain images.

Brigham Young University, Provo, Utah, USA
Correspondence regarding this page should be directed to Leigh Johnson at
Page copyright © 2004
 Page: Tree of Life
Polemoniaceae. Phlox Family.
Authored by
Leigh Johnson.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Polemoniaceae. Phlox Family.
Authored by
Leigh Johnson.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 22 April 2004
- Content changed 02 January 2009
Citing this page:
Johnson, Leigh. 2009. Polemoniaceae. Phlox Family. Version 02 January 2009. http://tolweb.org/Polemoniaceae/20812/2009.01.02 in The Tree of Life Web Project, http://tolweb.org/
















 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site