Lophiiformes
Anglerfishes
Theodore W. Pietsch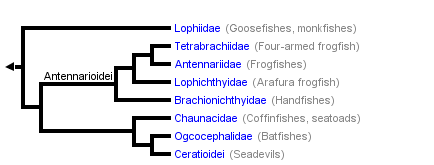


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The order Lophiiformes contains a highly diverse array of marine fishes that are primitively benthic shallow-water dwellers, but have evolved to form several groups of deep-shelf and slope inhabitants as well as a highly modified assemblage of open-water, meso- and bathypelagic species. Commonly referred to as anglerfishes, the group is characterized most strikingly by the structure of the first dorsal-fin spine, typically placed out on the tip of the snout and modified to serve as a luring apparatus.
The order contains approximately 322 living species, distributed among 65 genera and 18 families. The families themselves are distributed among five suborders (Pietsch, 1984a; Pietsch and Grobecker, 1987): the Lophioidei (reviewed by Caruso, 1981, 1983; Caruso and Bullis, 1976), containing a single family, four genera, and 25 species of relatively shallow-water, dorso-ventrally flattened forms, commonly referred to as the goosefishes or monkfishes; the Antennarioidei, with four families, 15 genera, and about 54 species, nearly all laterally compressed, shallow- to moderately deep-water, benthic forms, with a host of common names including frogfishes, sea-mice, sea-toads, warty anglerfishes, and handfishes (Pietsch, 1981, 1984a, 1984b; Last et al., 1983); the Chaunacoidei or coffinfishes, two genera and perhaps as many as 14 species (Caruso, 1989a, 1989b) of more or less globose, deep-water benthic forms; the Ogcocephaloidei or batfishes, a single family of ten genera and some 67 species of dorsoventrally flattened, deep-water benthic forms (Bradbury, 1967, 1980, 1988, 1999); and the Ceratioidei, the deep-sea anglerfishes, containing 11 families, 35 genera, and 162 species (Bertelsen, 1951, 1984; Pietsch, 1984a, 1999).
Characteristics
Anglerfishes display a wide range of body forms, from globose, almost spherical, to elongate, laterally compressed, or extremely dorsoventrally depressed. The head and mouth are typically large, the premaxillae protractile. The teeth in the jaws are numerous, small, villiform, in several rows, or very few in number and developed to form large fangs (as in most Ceratioidei). Vomerine teeth are usually present (absent in some Ceratioidei); palatine teeth are present or absent. The eyes are typically large (except in most adult female Ceratioidei). The anterior-most dorsal spine or illicium is nearly always present (but absent in male Ceratioidei and in both sexes of the ceratioid family Neoceratiidae), usually bearing a terminal bait or esca (absent in some Antennariidae, male Ceratioidei, and in both sexes of the ceratioid family Neoceratiidae). The esca is simple to highly complex, bioluminescent in nearly all female Ceratioidei. The bony support for the illicium (illicial pterygiophore), which lies within a shallow trough on the anterodorsal surface of the cranium, is highly protrusible in some taxa. The pectoral fins are highly modified, leg-like (except in Ceratioidei). When present, the pelvic fins are jugular in position and consist of 1 spine and 4 or 5 rays (pelvics are absent in Ceratioidei, except for larval Caulophrynidae). The gill openings are restricted to a small, elongate, tube-like opening situated immediately dorsal to, posterior to, or ventral to (rarely partly anterior to) the base of the pectoral fin. A pseudobranch is present or absent. A swimbladder is usually absent (present and physoclistous in some Antennariidae). The eggs are spawned in a double, scroll-shaped mucous sheath. The soft dorsal fin consists of 3-22 rays, the anal fin 3-19 rays, the pectoral fin 4-30 rays, and the caudal fin 8-10 rays.
The coloration of anglerfishes ranges from uniform gray, brown to black, without markings of any kind (e.g., some Lophioidei and Ceratioidei), to multicolored and complexly patterned (e.g., Antennariidae).
Typically small fishes, the largest known individuals of most families attain standard lengths of approximately 100-250 mm, but some (e.g., Lophiidae, some Antennariidae, Himantolophidae, Thaumatichthyidae, Ceratiidae, and Gigantactinidae) become much larger, lophiids exceeding a meter in length and a weight of 27 kg. Ceratioids display an extreme sexual dimorphism in which males are dwarfed, the largest known free-living individuals of most families measuring 10-30 mm SL (standard length), but reaching 40 mm SL in Himantolophidae; parasitically attached individuals usually range from about 7-30 mm SL, but reach nearly 120 mm SL in Ceratiidae.
List of Synapomorphies
- Spinous dorsal fin primitively of six spines, the anteriormost three of which are cephalic in position and modified to serve as a luring apparatus
- Epiotics separated from parietals and meeting on the midline posterior to the supraoccipital
- Gill opening restricted to a small, elongate, tube-like opening adjacent to pectoral-fin base
- A single hypural plate emanating from a single complex half-centrum
- Ventralmost pectoral radial considerably expanded distally
- Eggs spawned in a double scroll-shaped mucous sheath
- Posteromedial process of vomer emerging from ventral surface as a laterally compressed, keel-like structure
- Postmaxillary process of premaxilla spatulate
- Opercle reduced
- Ectopterygoid triradiate
- Proximal end of hypobranchials II and III bifurcate
- Interhyal with a medial, posterolaterally directed process
- Illicial pterygiophore and pterygiophore of third dorsal spine with highly compressed, blade-like dorsal expansions
- Eggs and larvae small
- Head of larvae large relative to body
- Number of dorsal-fin spines reduced
- Loss of pharyngobranchial IV
- Second dorsal spine reduced and embedded beneath skin of head
- Gill filaments of gill arch I absent
- Second dorsal spine reduced to a small remnant
- Third dorsal spine and pterygiophore absent
- Epibranchial I simple, without ligamentous connection to epibranchial II
- Posttemporal fused to cranium.
Geographic Distribution
With the exception of a single species of Antennariidae occasionally taken in brackish or even fresh water (Antennarius biocellatus; see Pietsch and Grobecker, 1987: 174), lophiiforms are strictly marine fishes distributed throughout all oceans and major seas of the world. Only the Lophiidae, however, are present in the Mediterranean. Most are benthic as adults, typically occupying depths that range from the surface down to approximately 200 m, a few species extending down to 2500 m or more. All Ceratioidei (with the exception of the thaumatichthyid genus Thaumatichthys, which is benthic in 1000-3600 m) are meso- and bathypelagic, concentrated between approximately 800 and 2500 m.
Reproduction and Early Life History
Little is known about the reproduction and early life history of lophiiform fishes (Breder and Rosen, 1966; Pietsch, 1976, 1984a; Martin and Drewry, 1978; Pietsch and Grobecker, 1987), detailed information being available for only a few members of the Lophiidae, Antennariidae, and most ceratioid families. Scattered bits of published data are also available for the Tetrabrachiidae, Brachionichthyidae, Chaunacidae, and Ogcocephalidae, but nothing has been reported for the Lophichthyidae.
Observations of courtship and spawning behavior have been reported for only a few antennariids. Eggs and larvae have been adequately described for two of the 25 known species of Lophiidae; larvae, but not eggs, have been described in a third species. Within the Antennariidae, unequal information concerning early life-history stages is available for only four of the 42 recognized species. For the Tetrabrachiidae and Brachionichthyidae, all that is published is a mention of egg attachment to dorsal-fin rays and substrate, respectively (Pietsch and Grobecker, 1980). For chaunacids and ogcocephalids, aside from brief descriptions of ovarian structure, limited (by available material) developmental series of an unidentified species of each of two genera (Chaunax and Ogcocephalus) were described by Pietsch (1984a). Finally, larvae, but not eggs, have been adequately described for most families of the Ceratioidei. For a full summary, see Pietsch (1984a), Pietsch and Grobecker (1987), and numerous references cited therein.
Probably the most striking characteristic of early ontogeny in lophiiform fishes is that eggs are spawned encapsulated within a non-adhesive, balloon-shaped mucoid mass (Ray, 1961) or, more typically, a continuous, ribbon-like sheath of gelatinous mucous, often referred to as an "egg-raft" or "veil" (with some exceptions, see Pietsch and Grobecker, 1980, 1987). These egg-rafts are complex structures of positive bouyancy that float freely at the surface. Each is a product of two confluent ovaries within which each individual egg floats in a separate chamber provided with openings for the circulation of water (Rasquin, 1958; Martin and Drewry, 1978, and numerous references cited therein). This peculiar structure, differing considerably from any other ovarian product known in fishes (Breder and Rosen, 1966), is an excellent device for broadcasting a large number of small eggs over great geographic distances providing for development in relatively productive surface waters (Gudger, 1905; Pietsch and Grobecker, 1980, 1987).
Economic Importance
Aside from lophiids, which are highly esteemed as food-fishes and utilized fresh, frozen, or for fishmeal and oil, lophiiforms have limited economic value and are generally not considered an exploitable source of animal protein. Antennariids, and to a smaller extent ogcocephalids, are utilized by the aquarium trade. Ceratioids have occasionally been recorded as food items for predatory fishes (e.g., Thunnus, and the lancetfishes Aphanopus carbo and Alepisaurus ferox; Maul, 1961, 1962; Matthews et al., 1977) and whales (e.g., sperm whales, Physeter catodon; Clarke, 1950, 1956; Penrith, 1967) but they are not known to constitute the principal or preferred diet of any species.
Relationship of Lophiiformes to Other Paracanthopterygii
The Lophiiformes has traditionally been allied with the order Batrachoidiformes. Regan (1912) initially believed these two groups to be so closely related that he included them as suborders of an order he called the "Pediculati." At that time Regan (1912:278) wrote: "The Batrachoidea are here included in the Pediculati rather than in the Percomorphi, for it can hardly be the case that the resemblance in osteological characters, especially in the structure of the pectoral arch, is not due to real affinity." But later Regan (1926:3) separated the Lophiiformes from the Batrachoidiformes, stating that "although the resemblances in the pectoral arch may be due to relationship, the differences in other characters are sufficient to keep them apart."
Since that time, Regan's (1926) revised opinion has been almost universally accepted. The more significant studies that reach this conclusion being those of Regan and Trewavas (1932), Gregory (1933), Gregory and Conrad (1936), Gregory (1951), Eaton et al. (1954), and Monod (1960). Greenwood et al. (1966) and Rosen and Patterson (1969) also agreed, summarizing the available supporting evidence and proposing further a close phylogenetic relationship between these two orders and the Gobiesociformes, these three taxa forming a so-called "Batrachoidiform Lineage" of the Paracanthopterygii. Since the publication of these two papers, Gosline (1970), in a long and detailed paper, removed the order Gobiesociformes from this lineage (and from the Paracanthopterygii), and recognized it, together with the Callionymidae and Draconettidae, as an order of perciform derivation. Without comment or new evidence, Lauder and Liem (1983) reversed Gosline's (1970) decision by reconstituting the "Batrachoidiform Lineage" as originally proposed by Rosen and Patterson (1969). Following Gosline (1970) in excluding the Gobiesociformes, Patterson and Rosen (1989: 24) once again reviewed all the available evidence and identified several new and significant characters in the anterior vertebrae and gill-arch skeleton. Of the batrachoidiforms and lophiiforms, they concluded that "the latter are surely monophyletic, the former less so, but if so, the two are sister groups."
Discussion of Phylogenetic Relationships
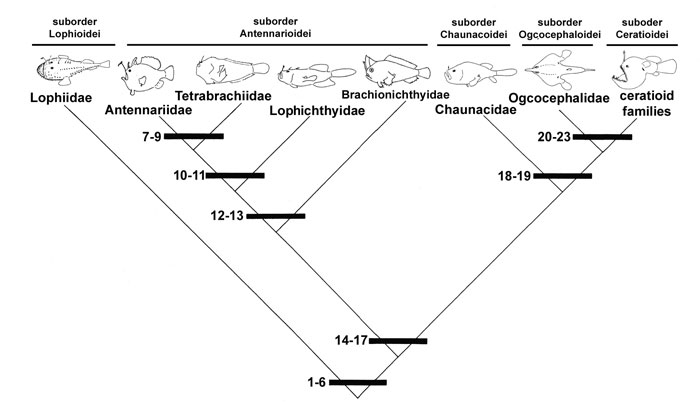
The relationships of the Lophiiformes as presented by Pietsch and Grobecker (1987). For character states, click here: 1-6, 7-9, 10-11, 12-13, 14-17, 18-19, and 20-23.
That the order Lophiiformes constitutes a natural assemblage seems certain. All included taxa share at least six unique and morphologically complex synapomorphic features (numbered 1 through 6 in the cladogram; a modification of Pietsch, 1981, 1984a):
- Spinous dorsal fin primitively of six spines, the anterior-most three of which are cephalic in position, the first modified to serve as a luring apparatus (involving numerous associated specializations, e.g., a medial depression of the anterior portion of the cranium, loss of the nasal bones and supraoccipital lateral-line commissure, and complex modifications of associated musculature and innervation);
- Epiotics separated from the parietals and meeting on the midline posterior to the supraoccipital;
- Gill opening restricted to a small, elongate, tube-like opening situated immediately dorsal to, posterior to, or ventral to (rarely partly anterior to) the pectoral-fin base;
- A single hypural plate emanating from a single complex half-centrum;
- Ventralmost pectoral radial considerably expanded distally (Pietsch, 1981: 397, 411, figs. 14, 40); and
- Eggs spawned in a double, scroll-shaped mucous sheath (Rasquin, 1958; Pietsch and Grobecker, 1987: 351).
Since Regan (1912), three major lophiiform taxa of equal rank have been recognized by nearly all authors. These taxa, together with their currently recognized families (the 11 families of the meso- and bathypelagic Ceratioidei excluded; see Bertelsen, 1951: 29; and Pietsch, 1972: 18), are as follows:
- Suborder Lophioidei
- Family Lophiidae
- Suborder Antennarioidei
- Family Antennariidae
- Family Tetrabrachiidae
- Family Lophichthyidae
- Family Brachionichthyidae
- Family Chaunacidae
- Family Ogcocephalidae
- Suborder Ceratioidei
Pietsch (1981: 416, fig. 41) attempted to test the validity of Regan's (1912) concept of three major lophiiform taxa by using cladistic analysis. In that study, serious difficulty was encountered in efforts to establish monophyly for the six families of Regan's (1912) Antennarioidei. Although a number of synapomorphic features were found to support a sister-group relationship between the four families Antennariidae through Brachionichthyidae, and between the families Chaunacidae and Ogcocephalidae, no convincing synapomorphy was found to link these two larger subgroups.
In a more recent attempt, Pietsch and Grobecker (1987: 268, fig. 110) proposed a new cladogram, which, in resolving the former difficulties, differed significantly from that previously published (Pietsch, 1981, fig. 41). In this revised cladogram, the suborder Antennarioidei is restricted to just four families: the Antennariidae, recognized as the sister-group of the Tetrabrachiidae, these two families together forming the sister-group of the Lophichthyidae, and this assemblage of three families forming the sister-group of the Brachionichthyidae. These relationships are supported by a total of seven synapomorphies, most of which were previously described by Pietsch (1981):
- Posteromedial process of the vomer emerging from the ventral surface as a laterally compressed, keel-like structure, its ventral margin (as seen in lateral view) strongly convex (1981: 397, figs. 4-6);
- Postmaxillary process of the premaxilla spatulate (1981: 398, figs. 8, 20);
- Opercle similarly reduced in size (1981: 401, figs. 9, 21);
- Ectopterygoid triradiate, a dorsal process overlapping the medial surface of the metapterygoid (1981: 400, figs. 9, 21, 22);
- Proximal end of hypobranchials II and III deeply bifurcate (1981: 407, figs. 11, 28, 29);
- Interhyal with a medial, posterolaterally directed process that makes contact with the respective preopercle (1981: 400, fig. 26);
- Illicial pterygiophore and pterygiophore of the third dorsal spine with highly compressed, blade-like dorsal expansions (1981: 410, figs. 36, 37).
The present interpretation of lophiiform relationships differs further from any previously proposed hypothesis in considering the Antennarioidei (sensu stricto) to form the primitive sister-group of a much larger group that includes the Chaunacoidei, the Ogcocephaloidei, and the Ceratioidei. The Ogcocephaloidei are in turn recognized as the primitive sister-group of the Ceratioidei.
Monophyly for a group containing the suborders Antennarioidei, Chaunacoidei, Ogcocephaloidei, and Ceratioidei is supported by four synapomorphies, all previously identified by Pietsch (1984a):
- Eggs and larvae small (at all stages the eggs are considerably less than 50% the diameter of those of lophioids; the smallest larvae are certainly less than 50%, and probably less than 30%, the size of those of lophioids; size at transformation to the prejuvenile stage is less than 60% that of lophioids; Pietsch, 1984a, fig. 164);
- Head of larvae proportionately large relative to the body (always greater than 45% SL, compared to less than 30% in lophioids; Pietsch, 1984a, fig. 164);
- Number of dorsal-fin spines reduced from a primitive six in lophioids to three or less (Pietsch, 1981: 409, figs. 36-38);
- Pharyngobranchial IV absent (present and well toothed in lophioids; Pietsch, 1981: 401, figs. 11, 28-32).
Monophyly for a group containing the suborders Chaunacoidei, Ogcocephaloidei, and Ceratioidei is supported by two synapomorphies:
- Second dorsal-fin spine reduced and embedded beneath skin of the head (Pietsch, 1981: 410, figs. 36-38);
- Gill filaments of gill arch I absent (but present on proximal end of ceratobranchial I of some ceratioids; Bradbury, 1967: 408; Pietsch, 1981: 415).
Monophyly for a group containing the Ogcocephaloidei and Ceratioidei is supported by four synapomorphies:
- Second dorsal-fin spine reduced to a small remnant (well developed in all other lophiiforms and secondarily developed in the ceratioid families Diceratiidae and Ceratiidae; Bertelsen, 1951: 17; Pietsch, 1981: 410, fig. 38);
- Third dorsal-fin spine and pterygiophore absent (present in all other lophiiforms; Bertelsen, 1951: 17; Bradbury, 1967: 401; Pietsch, 1981: 410, fig. 38);
- Epibranchial I simple, without ligamentous connection to epibranchial II (in batrachoidiforms and all other lophiiforms, epibranchial I bears a medial process ligamentously attached to the proximal tip of epibranchial II; Pietsch, 1981: 401, figs. 28-32);
- Posttemporal fused to cranium (attached to the cranium in batrachoidiforms and all other lophiiforms in such a way that considerable movement in an anterodorsal-posteroventral plane is possible; Pietsch, 1981: 411).
Of the possible cladograms that could be constructed on the basis of the data provided, the hypothesis presented is by far the most parsimonious (but see Pietsch, 1984a: 324, for a discussion of convergence or reversal of character states).
Key to the Major Subgroups of the Lophiiformes
The plesiomorphic and autapomorphic features of the major subgroups of the Lophiiformes are incorporated into the following analytical key:
- 1A. Postcephalic, spinous dorsal-fin of 1 to 3 spines; pharyngobranchial IV present; cleithrum with prominent posterior spine; subopercle with large ascending process attached to anterior margin of ventral rami of opercle; pseudobranch well developed; eggs and larvae large; head of larvae small relative to body (Suborder Lophioidei)
- 1B. Postcephalic, spinous dorsal-fin absent; pharyngobranchial IV absent; cleithral spine absent; subopercle with ascending process absent or reduced to a small projection detached from opercle; pseudobranch greatly reduced or absent; eggs and larvae small; head of larvae large relative to body (go to 2)
- 2A. Spinous dorsal of 3 spines emerging from dorsal surface of cranium; illicial pterygiophore and pterygiophore of third dorsal-fin spine with highly compressed, blade-like dorsal expansions; interhyal with a medial, posterolaterally directed process that comes into contact with the respective preopercle; interopercle flat and broad (Suborder Antennarioidei, go to 3)
- 2B. Spinous dorsal of 2 or 3 spines, but only anteriormost spine emerging from dorsal surface of cranium (spines II and III reduced and embedded beneath skin of head or lost); illicial pterygiophore and pterygiophore of third dorsal-fin spine without blade-like dorsal expansions; interhyal without a medial, posterolaterally directed process; interopercle elongate and narrow (go to 6)
- 3A. Parietals meeting on the midline dorsal to supraoccipital; ectopterygoid roughly oval in shape or absent; ceratobranchials I through III with 1 or more tooth-plates; hypobranchial II simple, hypobranchial III absent; pectoral radials 2; pelvic fin of 1 spine and 4 rays (Family Brachionichthyidae)
- 3B. Parietals well separated by supraoccipital; ectopterygoid triradiate, a dorsal process overlapping medial surface of metapterygoid; ceratobranchials I through IV toothless; hypobranchials II and III bifurcate proximally; pectoral radials 3; pelvic fin of 1 spine and 5 rays (go to 4)
- 4A. Vomer wide, the width between lateral ethmoids nearly as great as that between lateral margins of sphenotics; vomer without posteromedial process; dorsal head of quadrate broad, the width equal to or greater than that of metapterygoid; postmaxillary process of premaxilla tapering to a point; opercle expanded posteriorly; pharyngobranchial and epibranchial of first arch toothed; bony connection between tips of haemal spines of 14th through 16th preural centra; pterygiophore of illicium elongate, greatly depressed and laterally expanded posteriorly (Family Lophichthyidae)
- 4B. Vomer narrow, the width between lateral ethmoids considerably less than that between lateral margins of sphenotics; posteromedial process of vomer emerging from ventral surface as a laterally compressed, keel-like structure, its ventral margin (as seen in lateral view) strongly convex; dorsal head of quadrate narrow, the width less than that of metapterygoid; postmaxillary process of premaxilla spatulate; opercle reduced in size; pharyngobranchial and epibranchial of first arch toothless; bony connection between tips of haemal spines absent; pterygiophore of illicium short, the posterior end cylindrical (go to 5)
- 5A. Eyes dorsal; dorsal-fin spines reduced; mouth small; pectoral fin double, the dorsal-most ray of ventral portion membranously attached to side of body; pectoral-fin lobe membranously attached to rays of pelvic fin; soft-dorsal fin rays 16 or 17, anal fin rays 11 or 12 (Family Tetrabrachiidae)
- 5B. Eyes lateral; dorsal-fin spines well developed; mouth large; pectoral fin single, the rays not membranously attached to side of body; pectoral-fin lobe not membranously attached to rays of pelvic fin; soft-dorsal fin rays 11 to 15, anal fin rays 6 to 9 ( Family Antennariidae)
- 6A. Second dorsal-fin spine elongate, embedded beneath skin of head; third dorsal spine and pterygiophore present; epibranchial I with a medial process ligamentously attached to proximal tip of epibranchial II ( Suborder Chaunacoidei)
- 6B. Second dorsal-fin spine reduced to a tiny remnant embedded beneath skin of head and lying on, or fused to, dorsal surface of pterygiophore just behind base of illicial bone; third dorsal spine and pterygiophore absent; epibranchial I simple, without ligamentous attachment to epibranchial II (go to 7)
- 7A. Ceratobranchial V toothed, expanded proximally; pelvic fins present; obvious sexual dimorphism absent, the males not dwarfed (Suborder Ogcocephaloidei)
- 7B. Ceratobranchial V toothless, reduced to a slender rod-shaped element; pelvic fins absent (except in larval caulophrynids); sexual dimorphism strongly developed, the males dwarfed to a small fraction of size of females (Suborder Ceratioidei)
Fossil Lophiiforms
Patterson and Rosen (1989: 23) summarized what little is known about fossil lophiiforms. The record extends back to the early Pliocene of Algeria (Lophius budegassa Arambourg), but better represented in the early Eocene of Monte Bolca (Eastman, 1904), where Blot (1980) said that a new genus of lophiid is to be named for Lophius brachysomus Agassiz; that a new genus of ogcocephalid is to be described; and that Histionotophorus bassanii (de Zigno) is an antennariid. Blot (1980) did not cite any reasons for placing Histionotophorus in the Antennariidae, and did not cite Rosen and Patterson's (1969) brief discussion of the genus, which they placed in the antennarioid family Brachionichthyidae. Pietsch (1981: 416) agreed, and suggested that the Eocene genus is synonymous with the extant Brachionichthys Bleeker. Thus the Monte Bolca lophiiforms indicate that at least three of the major lophiiform lineages (lophioids, antennarioids, and ogcocephaloids) were already in existence in the early Eocene. The only known ceratioid fossil, a female specimen identified as Acentrophryne longidens Regan (family Linophrynidae), was described from the Late Miocene of California by Pietsch and Lavenberg (1980).
References
Bertelsen, E. 1951. The ceratioid fishes. Ontogeny, taxonomy, distribution and biology. Dana Rept., 39, 276 pp.
Bertelsen, E. 1984. Ceratioidei: Development and relationships. pp. 325-334, In: Moser, H. G., W. J. Richards, D. M. Cohen, M. P. Fahay, A. W. Kendall, Jr., and S. L. Richardson (editors), Ontogeny and Systematics of Fishes, Spec. Publ. No. 1, Amer. Soc. Ichthy. Herpet., ix + 760 pp.
Blot, J. 1980. La faune ichthyologique des gisements du Monte Bolca (Province de Verone, Italie). Bull. Mus. Nat. Hist. Nat., Paris, Ser. 4, 2, C: 339-396.
Bradbury, M. G. 1967. The genera of batfishes (family Ogcocephalidae). Copeia, 1967(2): 399-422.
Bradbury, M. G. 1980. A revision of the fish genus Ogcocephalus with descriptions of new species from the Western Atlantic Ocean (Ogcocephalidae: Lophiiformes). Proc. Calif. Acad. Sci., 42(7): 229-285.
Bradbury, M. G. 1988. Rare fishes of the deep-sea genus Halieutopsis: A review with descriptions of four new species (Lophiiformes: Ogcocephalidae). Fieldiana, Zoology, n.s., 44, 22 pp.
Bradbury, M. G. 1999. A review of the fish genus Dibranchus, with descriptions of new species and a new genus, Solocisquama (Lophiiformes: Ogcocephalidae). Proc. Calif. Acad. Sci., 15(5): 259-310.
Breder, C. M., and D. E. Rosen. 1966. Modes of Reproduction in Fishes. Natural History Press, Garden City, N.Y., 941 pp.
Caruso, J. H. 1981. The systematics and distribution of the lophiid anglerfishes: I. A revision of the genus Lophiodes, with the description of two new species. Copeia, 1981(3): 522-549.
Caruso, J. H. 1983. The systematics and distribution of the lophiid anglerfishes: II. Revisions of the genera Lophiomus and Lophius. Copeia, 1983(1): 11-30.
Caruso, J. H. 1985. The systematics and distribution of the lophiid anglerfishes: III. Intergeneric relationships. Copeia, 1985(4): 870-875.
Caruso, J. H. 1989a. Systematics and distribution of the Atlantic chaunacid anglerfishes (Pisces: Lophiiformes). Copeia, 1989(1): 153-165.
Caruso, J. H. 1989b. A review of the Indo-Pacific members of the deep-water chaunacid anglerfish genus Bathychaunax, with the description of a new species from the Eastern Indian Ocean (Pisces: Lophiiformes). Bull. Mar. Sci., 45(3): 574-579.
Caruso, J. H., and H. R. Bullis, Jr. 1976. A review of the lophiid anglerfish genus Sladenia with a description of a new species from the Caribbean Sea. Bull. Mar. Sci., 26(1): 59-64.
Clarke, R. 1950. The bathypelagic angler fish Ceratias holboelli Krøyer. Disc. Rept., 26: 1-32.
Clarke, R. 1956. Sperm whales of the Azores. Disc. Rept., 28: 237-298.
Eastman, C. R. 1904. Descriptions of Bolca fishes. Bull. Mus. Comp. Zool., Harvard, 46(1): 1-36.
Eaton, T. H., Jr., C. A. Edwards, M. A. McIntosh, and J. P. Rowland. 1954. Structure and relationships of the anglerfish, Lophius americanus. J. Elisha Mitchell Sci. Soc., 70(2): 205-218.
Gosline, W. A. 1970. A reinterpretation of the teleostean order Gobiesociformes. Proc. Calif. Acad. Sci., Ser. 4, 37(19): 363-382.
Greenwood, P. H., D. E. Rosen, S. H. Weitzman, and G. S. Myers. 1966. Phyletic studies of teleostean fishes, with a provisional classification of living forms. Bull. Amer. Mus. Nat. Hist., 131: 339-456.
Gregory, W. K. 1933. Fish skulls: A study of the evolution of natural mechanisms. Trans. Amer. Phil. Soc., 23(2): 75-481.
Gregory, W. K. 1951. Evolution Emerging: A Survey of Changing Patterns from Primeval Life to Man. The Macmillan Company, New York, Vol. 1, 704 pp.
Gregory, W. K., and G. M. Conrad. 1936. The evolution of the pediculate fishes. Amer. Nat., 70(728): 193-208.
Gudger, E. W. 1905. A note on the eggs and egg laying of Pterophryne histrio, the gulfweed fish. Science, N.Y., n.s., 22: 841-843
Last, P. R., E. O. G. Scott, and F. H. Talbot. 1983. Fishes of Tasmania. Tasmanian Fisheries Development Authority, Hobart, viii + 563 pp.
Lauder, G. V., and K. F. Liem. l983. The evolution and interrelationships of the actinopterygian fishes. Bull. Mus. Comp. Zool., Harvard, 150(3): 95-197.
Martin, F. D., and G. E. Drewry. 1978. Development of Fishes of the Mid-Atlantic Bight: An Atlas of Eggs, Larvae, and Juvenile Stages. Vol. 6. Stromateidae through Ogcocephalidae. Biol. Ser. Prog., Fish Wildl. Serv., U.S. Dept. Interior, 416 pp.
Matthews, F. D., D. M. Damkaer, L. W. Knapp, and B. B. Collette. 1977. Food of Western North Atlantic tunas (Thunnus) and lancetfishes (Alepisaurus). NOAA Techn. Rept. NMFS SSRF-706, 19 pp.
Maul, G. E. 1961. The ceratioid fishes in the collection of the Museu Municipal do Funchal (Melanocetidae, Himantolophidae, Oneirodidae, Linophrynidae). Bol. Mus. Mun. Funchal, 14(50): 87-159.
Maul, G. E. 1962. On a small collection of ceratioid fishes from off Dakar and two recently acquired specimens from stomachs of Aphanopus carbo taken in Madeira (Melanocetidae, Himantolophidae, Diceratiidae, Oneirodidae, Ceratiidae). Bol. Mus. Mun. Funchal, 16(54): 5-27.
Monod, T. 1960. A propos du pseudobrachium des Antennarius (Pisces, Lophiiformes). Bull. Inst. Fr. Afr. Noire, 22, Ser. A, 2: 620-698.
Patterson, C., and D. E. Rosen. 1989. The Paracanthopterygii revisited: Order and disorder. pp. 5-36, In: Papers on the Systematics of Gadiform Fishes, D. M. Cohen (editor), Natural History Museum of Los Angeles County, Sci. Ser. 32.
Penrith, M. J. 1967. Ceratioid angler-fishes from South Africa. J. Nat. Hist., 1: 185-188.
Pietsch, T. W. 1972. A review of the monotypic deep-sea anglerfish family Centrophrynidae: Taxonomy, distribution, and osteology. Copeia, 1972(1): 17-47.
Pietsch, T. W. 1976. Dimorphism, parasitism and sex: reproductive strategies among deepsea ceratioid anglerfishes. Copeia, 1976(4): 781-793.
Pietsch, T. W. 1981. The osteology and relationships of the anglerfish genus Tetrabrachium, with comments on lophiiform classification. U. S. Fish. Bull., 79(3): 387-419.
Pietsch, T. W. 1984a. Lophiiformes: Development and relationships. pp. 320-325, In: Moser, H. G., W. J. Richards, D. M. Cohen, M. P. Fahay, A. W. Kendall, Jr., and S. L. Richardson (editors), Ontogeny and Systematics of Fishes, Spec. Publ. No. 1, Amer. Soc. Ichthy. Herpet., ix + 760 pp.
Pietsch, T. W. 1984b. The genera of frogfishes (family Antennariidae). Copeia, 1984(1):27-44.
Pietsch, T. W. 1999. [Caulophrynidae, Neoceratiidae, Melanocetidae, Himantolophidae, Diceratiidae, Oneirodidae, Thaumatichthyidae, Centrophrynidae, Ceratiidae, Gigantactinidae, and Linophrynidae]. pp. 2026-2037, In: FAO Species Identification Sheets for Fishery Purposes. Western Central Pacific (Fishing Area 71 and the southwestern part of Area 77). Food and Agriculture Organization of the United Nations, Rome. Vol. 3, Batoid fishes, Chimaeras, and Bony fishes Part 1 (Elopidae to Linophrynidae).
Pietsch, T. W., and D. B. Grobecker. 1980. Parental care as an alternative reproductive strategy in antennariid anglerfishes. Copeia, 1980(3): 551-553.
Pietsch, T. W., and D. B. Grobecker. 1987. Frogfishes of the World: Systematics, Zoogeography, and Behavioral Ecology. Stanford University Press, Stanford, xxii + 420 pp.
Pietsch, T. W., and R. J. Lavenberg. 1980. A fossil ceratioid anglerfish from the Late Miocene of California. Copeia, 1980(4): 906-908.
Rasquin, P. 1958. Ovarian morphology and early embryology of the pediculate fishes Antennarius and Histrio. Bull. Amer. Mus. Nat. Hist., 11(4): 331-371.
Ray, C. 1961. Spawning behavior and egg raft morphology of the ocellated fringed frogfish, Antennarius nummifer (Cuvier). Copeia, 1961(2): 230-231.
Regan, C. T. 1912. The classification of the teleostean fishes of the order Pediculati. Ann. Mag. Nat. Hist., Ser. 8, 9(28): 277-289.
Regan, C. T. 1926. The pediculate fishes of the suborder Ceratioidea. Dana Oceanogr. Rept. 2, 45 pp.
Regan, C. T., and E. Trewavas. 1932. Deep-sea anglerfish (Ceratioidea). Dana Rept., 2, 113 pp.
Rosen, D. E., and C. Patterson. 1969. The structure and relationships of the paracanthopterygian fishes. Bull. Amer. Mus. Nat. Hist., 141: 357-474.
Title Illustrations

| Scientific Name | Lophius piscatorius Linnaeus |
|---|---|
| Acknowledgements | Courtesy of Kåre Telnes |
| Specimen Condition | Live Specimen |
| Copyright |
© 2005 Kåre Telnes

|
About This Page
Theodore W. Pietsch

University of Washington, Seattle, Washington, USA
Correspondence regarding this page should be directed to Theodore W. Pietsch at and Christopher P. Kenaley at
Page copyright © 2005 Theodore W. Pietsch
 Page: Tree of Life
Lophiiformes. Anglerfishes.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Lophiiformes. Anglerfishes.
Authored by
Theodore W. Pietsch.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 18 October 2005
Citing this page:
Pietsch, Theodore W. 2005. Lophiiformes. Anglerfishes. Version 18 October 2005 (under construction). http://tolweb.org/Lophiiformes/21989/2005.10.18 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site