Ceratioidei
Seadevils, Devilfishes, Deep-sea Anglerfishes
Theodore W. Pietsch and Christopher P. Kenaley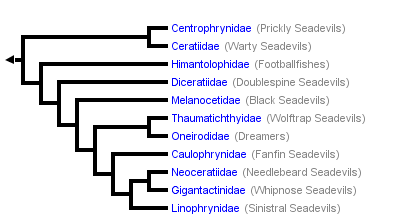


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Of the five major taxa of lophiiform fishes, the deep-sea Ceratioidei is the most phylogenetically derived (Bertelsen, 1984; Pietsch, 1984). Ceratioidei are distributed throughout the world’s oceans below a depth of 300 m. With 160 species, it constitutes by far the most species-rich vertebrate taxon within the bathypelagic zone and below—more than twice as many families and genera and more than three times the number of species as the Cetomimoidei, the next most species-rich deep-sea vertebrate taxon (see Paxton, 1998; Herring, 2002). At the same time, new species are being added to the suborder at a steady if not increasing rate.
Members of the group differ remarkably from their less-derived, bottom-living relatives by having an extreme sexual dimorphism (shared by all contained taxa) and a unique mode of reproduction in which the males are dwarfed—those of some linophrynids, adults at 6-10 mm standard length, competing for the title of world’s smallest vertebrates (see Winterbottom and Emery, 1981; Roberts, 1986; Weitzman and Vari, 1988; Kottelat and Vidthayanon, 1993; Watson and Walker, 2004)—and attach themselves (either temporarily or permanently) to the bodies of relatively gigantic females. In Ceratias holboelli, where the most extreme examples are found, females may be more than 60 times the length and about a half a million times as heavy as the males (Bertelsen, 1951; Pietsch, 1976, 1986). The males lack a luring apparatus and those of most species are equipped with large well-developed eyes (Munk, 1964, 1966) and relatively huge nostrils (Marshall, 1967a, b), the latter apparently used for homing in on a female-emitted, species-specific pheromone (Bertelsen, 1951; Pietsch, 1976; Munk, 1992). Normal jaw teeth are lost during metamorphosis, but are replaced by a set of pincer-like denticles at the anterior tips of the jaws for grasping and holding fast to a prospective mate. In some taxa male attachment becomes permanent and parasitic through fusion of male and female tissue (see Sexual Parasitism in Ceratioid Anglerfishes).
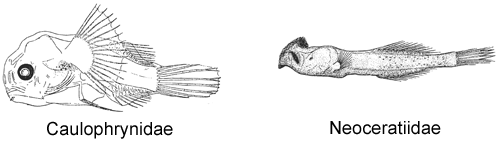
Ceratioid anglerfishes differ further from their shallow-water relatives in having a bacterial light-organ that serves as bait to attract prey, a structure technically called the “esca”—exceptions among members of the suborder include the monotypic family Neoceratiidae (Bertelsen, 1951), the three species of the gigantactinid genus Rhynchactis (Bertelsen et al., 1981; Bertelsen and Pietsch, 1998), and the five members of the family Caulophrynidae (Pietsch, 1979). Parr (1927) was the first to recognize the diagnostic value of the external morphology of escae in ceratioids, pointing out the need for a closer examination of individual variation in the structure of this organ. Since that time, differences in the number, shape, and size of escal appendages and filaments, as well as variation in external escal pigment patterns, have been, for the most part, the sole basis on which new species have been described (e.g., see Pietsch, 1974; Bertelsen et al., 1981; Bertelsen and Krefft, 1988).
The internal structure of ceratioid escae is infinitely more complex, involving a confusing array of bacteria-filled vesicles, light-absorbing pigment layers, reflecting tissues, tubular light-guiding structures, nerves, blood vessels, and smooth muscle fibers (Munk and Bertelsen, 1980; Munk, 1988, 1998, 1999; Herring and Munk, 1994; Munk and Herring, 1996; Munk et al., 1998). There is some evidence also that ceratioid escae contain pheromone-producing secretory glands that function to attract a conspecific male (Munk, 1992), but the true nature and adaptive significance of these structures and most of the other internal parts of escae are unknown.
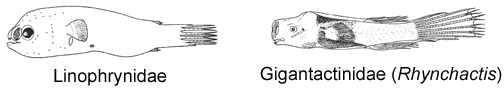
In addition to the esca, all 23 currently recognized species of the ceratioid genus Linophryne (family Linophrynidae) bear an elaborate bioluminescent hyoid barbel, the light of which does not originate from symbiotic luminescent bacteria but rather from a complex array of intrinsic, intracellular, paracrystalline photogenic granules; the bacteria-filled esca is ectodermal in origin, whereas the barbel light organ appears to be derived from the mesoderm (Hansen and Herring, 1977). This remarkable dual system, involving two entirely separate mechanisms of light production, is unique among animals.
In summary, ceratioid anglerfishes are among the most intriguing of all animals, possessing a host of spectacular morphological, behavioral, and physiological innovations found nowhere else. The suborder is taxonomically diverse: with 160 currently recognized species (and many more certain to be discovered in the future), it forms a major contribution to the biodiversity of the deep-sea. It is exceedingly widespread geographically, occurring in deep waters of all major oceans and seas of the world, from high Arctic latitudes to the Southern Ocean; while some species appear to be almost cosmopolitan in distribution, many others have surprisingly small, restricted, vertical and horizontal ranges. Their relative abundance, high species diversity, and trophic position as the top primary carnivores in meso- and bathypelagic communities make them important ecologically. Their unique mode of reproduction has significant biomedical implications to the fields of endocrinology and immunology. Yet, despite these many aspects of biological interest and importance, as well as a large amount of revisionary work published in the 1970s and early 1980s, including repeated attempts to resolve phylogenetic relationships, ceratioid anglerfishes have remained poorly known.
Characteristics
Diagnosis
A suborder of lophiiforms unique and derived in lacking pelvic fins (except in larval and newly metamorphosed Caulophrynidae), pelvic bones reduced or absent; extremely sexually dimorphic, males dwarfed, a fraction of size of females. Spinous dorsal-fin of two spines (the illicium and second cephalic spine), both supported by a single pterygiophore; anteriormost dorsal-fin spine modified to serve as a lure, emerging from dorsal surface of cranium (somewhat behind cranium in Bufoceratias, from roof of mouth in Thaumatichthyidae, absent in Neoceratiidae); second dorsal-fin spine reduced to a tiny remnant (but relatively well developed in adolescent Diceratiidae and Ceratiidae, absent in Neoceratiidae and Linophrynidae), embedded beneath skin of head and lying on, or fused to, dorsal surface of pterygiophore just behind base of illicial bone; postcephalic, spinous dorsal-fin absent; palatine teeth absent; interhyal without medial, posterolaterally directed process; ceratobranchial V toothless, reduced to a slender rod-shaped element in most families, represented by tiny remnants in the Gigantactinidae; epibranchial I simple, without ligamentous attachment to epibranchial II; pharyngobranchial IV absent; cleithral spine absent; interopercle reduced, elongate and narrow; subopercle with ascending process absent or reduced to a small spine or projection detached from opercle; gill filaments present as holobranchs on gill arches II and III, extending as hemibranchs onto proximal end of arch I in some families (i.e., Caulophrynidae, Himantolophidae, Melanocetidae, Diceratiidae, some Oneirodidae, Centrophrynidae, and Neoceratiidae), and as hemibranchs on arch IV; pseudobranch absent (tiny remnants present in some Gigantactis); swimbladder absent.
Males with elements of cephalic dorsal-fin spines greatly reduced, remnants embedded beneath skin of head; eyes and olfactory organs greatly enlarged in most taxa; tip of snout and chin bearing hooked denticular teeth, used for attachment to females; in some families and genera attachment becomes parasitic through fusion of male and female tissue.
Description
Females with body short and deep, almost spherical in most families, but elongate and somewhat laterally compressed in Thaumatichthyidae, Centrophrynidae, Ceratiidae, Gigantactinidae, Neoceratiidae, and some Oneirodidae; head length usually greater than 40% SL, but only about 25% in elongate species; mouth moderate to extremely large (compared to other lophiiforms), length of premaxilla as little as 10% SL (in some Gigantactinidae) to as great as 40% SL (in some Linophrynidae); cleft of mouth nearly horizontal (e.g., Thaumatichthyidae, Centrophrynidae, and Gigantactinidae) or oblique to almost vertical (e.g., Melanocetidae and Ceratiidae).
Sexual dimorphism extreme: males dwarfed, those of most genera reaching a maximum standard length of only 6-8% of that of females, and less than 25% of that of the largest females of genera for which mature females are known; body elongate, head less than 40% SL; mouth small, length of premaxilla usually less than 15% SL; cleft of mouth horizontal. Metamorphosed males of all families with hooked denticular teeth apparently adapted especially for gripping the skin of females; becoming permanently attached and parasitic in Caulophrynidae, Ceratiidae, Neoceratiidae, Linophrynidae, and the oneirodid genera Bertella and Leptacanthichthys.
Fin-ray counts the same for males and females, highly variable: Dorsal rays 3-8 in most families, 11-13 in Neoceratiidae, and 12-22 in Caulophrynidae and Melanocetidae; anal rays 3-7 in most families, 5-19 in Caulophrynidae, and 10-13 in Neoceratiidae; caudal rays nearly always 9, but 8 in the ceratiid genus Cryptopsaras and 10 in some specimens of Neoceratiidae; pectoral rays 12-30. Pelvic fins absent (except in larval and early metamorphosis stages of Caulophrynidae). Rays of unpaired fins sometimes greatly elongate (e.g., in females of Caulophrynidae and Gigantactinidae) and often not interconnected, or interconnected with extremely thin, transparent, membrane-like tissue. Caudal fin rounded in nearly all families, but emarginate in some Gigantactinidae; caudal rays of some large individuals of Ceratiidae terminating in spherical skin-covered ossifications.
Coloration of males and females usually uniform dark brown to black over entire head, body, and fins; dermis everywhere unpigmented and translucent in the linophrynid genus Haplophryne.
Sexual Parasitism in Ceratioid Anglerfishes
In some ceratioid taxa, the male's attachment to the female is followed by fusion of epidermal and dermal tissues and, eventually, by a connection of the circulatory systems so that the male becomes permanently dependent on the female for blood-transported nutrients, while the host female becomes a kind of self-fertilizing hermaphrodite (Regan, 1925a, b, 1926; Parr, 1930; Regan and Trewavas, 1932; Bertelsen, 1951; Pietsch, 1975, 1976; Munk and Bertelsen, 1983; Munk, 2000). Permanent attachment is usually accomplished by means of separate outgrowths from the snout and tip of the lower jaw of the male, both of which eventually fuse with the skin of the female. In some species a papilla of female tissue protrudes into the mouth of the male, sometimes appearing to completely occlude the pharynx. The heads of some males become broadly fused to the skin of the female, extending from the tip of the lower jaw to the rear of the skull, appearing as if embedded or absorbed by their mate, while in others, the male is carried at the tip of an elongate, cylindrical stalk of female tissue.
Increasing considerably in size once fused, their volume becoming much greater than free-living males of the same species, and being otherwise completely unable to acquire nutrients on their own, the males are considered to be parasites. They apparently remain alive and reproductively functional as long as the female lives, participating in repeated spawning events. A single male per female appears to be the rule in some taxa, but in others multiple attachments are relatively common, with as many as eight coupled to a single host (Saruwatari et al., 2001).
Since its discovery some 80 years ago (Saemundsson, 1922; Regan, 1925a, b), the story of sexual parasitism in ceratioid anglerfishes has become a part of common scientific knowledge. However, the known facts concerning this remarkable reproductive mode have never been thoroughly and satisfactorily analyzed, despite the work of Bertelsen (1951) and more recently of Munk and Bertelsen (1983), Munk (2000), and Pietsch (2005). The physiological mechanisms (endocrinological and immunological) that allow for sexual parasitism, which could be of significant biomedical importance, have never been explored.
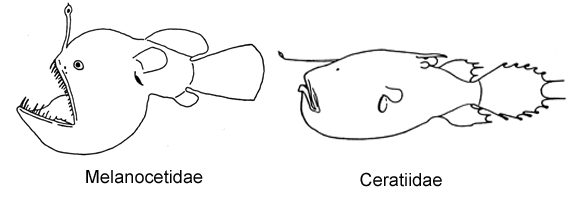
Key to Known Families of the Suborder Ceratioidei
Separate keys are provided for females and males.
Females
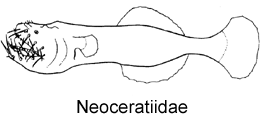
1A. Illicium absent; numerous elongate mobile teeth situated on outer margins of jaws (Neoceratiidae)
1B. Illicium present; outer margins of jaws without teeth (go to 2)
2A. Illicium without bulbous distal light organ, terminating with or without slender filaments (go to 3)
2B. Illicium with bulbous light organ at distal tip (go to 4)
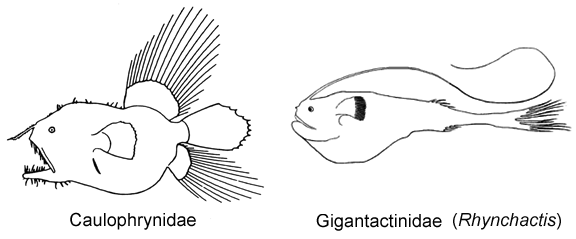
3A. Longest rays of dorsal and anal fin greater than 60% SL; body short, globose; mouth large, lower jaw usually extending posteriorly well beyond base of pectoral-fin lobe; upper and lower jaws well toothed (Caulophrynidae)
3B. Longest rays of dorsal and anal fin much less than 60% SL; body elongate, fusiform; mouth small, lower jaw terminating well before base of pectoral-fin lobe; upper and lower jaws nearly toothless (Gigantactinidae, genus Rhynchactis)
4A. Dorsal fin with more than 11 rays (Melanocetidae)
4B. Dorsal fin with fewer than 11 rays (go to 5)
5A. Two or three club-shaped caruncles on dorsal midline just anterior to soft dorsal fin; cleft of mouth vertical to strongly oblique (Ceratiidae)
5B. Dorsal midline without caruncles; cleft of mouth nearly horizontal (go to 6)
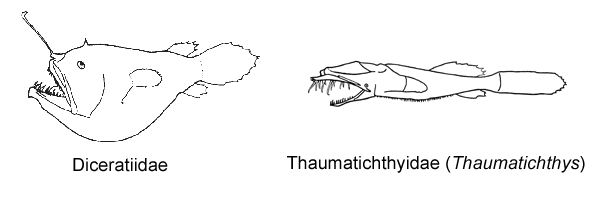
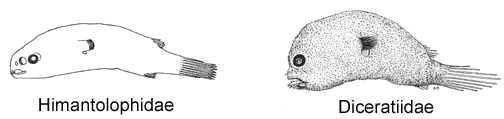
6A. A second cephalic ray immediately posterior to base of illicium, bearing a distal luminous gland, withdrawn beneath skin in larger specimens, its presence indicated by a small pore (Diceratiidae)
6B. Illicial apparatus without an external second cephalic ray; small pore posterior to base of illicium absent (go to 7)

7A. Upper jaw extending anteriorly far beyond lower jaw; esca with 1-3 bony denticles (Thaumatichthyidae)
7B. Jaws equal anteriorly; esca without bony denticles (go to 8)
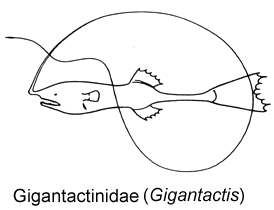
© 2005 Theodore W. Pietsch
8A. Illicium emerging from anteriormost tip of snout; length of head less than 35% SL; length of caudal peduncle more than 20% SL (Gigantactinidae, genus Gigantactis)
8B. Illicium emerging from behind tip of snout; length of head more than 35% SL; length of caudal peduncle less than 20% SL (go to 9)
9A. Skin more or less covered with dermal spinules or plates (go to 10)
9B. Skin naked (although microscopic dermal spinules may be present, skin appearing naked and smooth) (go to 11)
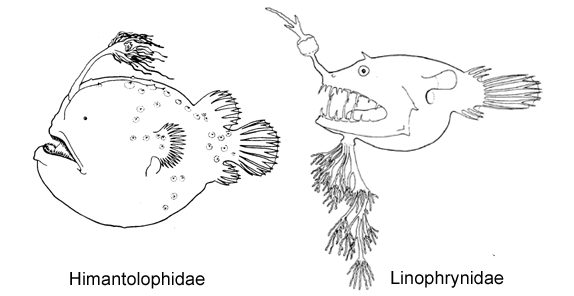
10A. Skin with some large bony plates, each bearing a median spine (Himantolophidae, females greater than 30-40 mm SL)
10B. Skin covered with numerous close-set dermal spinules (go to 12)
11A. Branchiostegal rays 4-5; dorsal-fin rays 3 (very rarely 2 or 4); anal rays 3 (Linophrynidae)
11B. Branchiostegal rays 6; dorsal-fin rays more than 4; anal-fin rays 4-7 (go to 13)
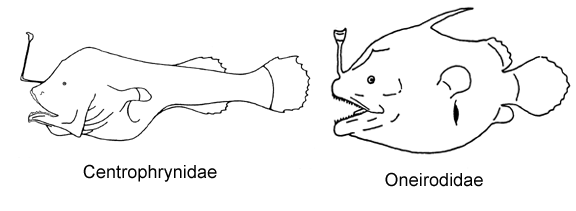
12A. Three pectoral radials; ceratobranchials toothless; branchiostegal rays narrow and cylindrical (Oneirodidae, genus Spiniphryne)
12B. Four pectoral radials; ceratobranchials well toothed; branchiostegal rays broad and laterally compressed (Centrophrynidae)
13A. Snout and chin rounded and blunt; bones of lower jaw enlarged and expanded ventrally, chin large, protruding anteriorly beyond snout (Himantolophidae, females less than 30-40 mm SL)
13B. Snout and chin more or less pointed; bones of lower jaw narrow; chin small, more or less equal to anterior extent of snout (Oneirodidae)
Males
1A. Anal-fin rays more than 9; upper denticular teeth absent (go to 2)
1B. Anal-fin rays fewer than 9; upper denticular teeth present (go to 3)
2A. Lower denticular with approximately 9 teeth; ventral fins present in larval and early metamorphic stages; dorsal-fin rays 14-22; anal-fin rays 13-19 (Caulophrynidae)
Males of the caulophrynid genus Robia, the single known female of which has dorsal-fin rays 6 and anal-fin rays 5, are unknown.
2B. Lower denticular trifurcate, each branch with a double hook; ventral fins absent; dorsal-fin rays 11-13; anal-fin rays 10-13 (Neoceratiidae)
3A. Olfactory organs small; eyes large, bowl-shaped (Ceratiidae)
3B. Olfactory organs large; eyes not bowl-shaped (go to 4)
4A. Dorsal-fin rays more than 11 (Melanocetidae)
4B. Dorsal-fin rays fewer than 11 (go to 5)5A. Dorsal-fin rays fewer than 5 (go to 6)
5B. Dorsal-fin rays 5-8 (go to 7)
6A. Eyes slightly tubular, directed more or less anteriorly; dorsal- and anal-fin rays 3 (rarely 2 or 4) (Linophrynidae)
6B. Eyes spherical, not tubular, directed laterally; dorsal-fin rays 4 (rarely 3), anal-fin rays 3-4 (Gigantactinidae, genus Rhynchactis)
7A. Eyes small, diameter 5% SL or less (go to 8)
7B. Eyes large, diameter greater than 5% SL (go to 9)
8A. A small digitiform hyoid barbel; bases of denticular teeth fused to form dorsal and ventral denticular tooth plates (Centrophrynidae)
8B. Hyoid barbel absent; all bases of denticular teeth mutually free, not forming discrete tooth plates (Gigantactinidae)
9A. Skin completely covered with well-developed dermal spinules; anterior nostrils opening laterally (go to 10)
9B. Skin naked, dermal spinules absent or extremely small and scattered; anterior nostrils opening anteriorly near end of snout (go to 11)
10A. Tip of snout with more than 10 denticular teeth, all fused at base (Himantolophidae)
10B. Tip of snout with two separate denticular teeth (Diceratiidae)
11A. Skin with small, but distinct dermal spinules scattered over surface of body (Thaumatichthyidae)
Males of the thaumatichthyid genus Lasiognathus, the females of which have naked skin, are unknown.
11B. Skin naked or appearing so, dermal spinules absent or microscopic in size (Oneirodidae)
Males of eight of the 16 recognized oneirodid genera are unknown, including Spiniphryne, the females of which have spinulose skin.
References
Bertelsen, E. 1951. The ceratioid fishes. Ontogeny, taxonomy, distribution and biology. Dana Rept., 39, 276 pp.
Bertelsen, E. 1984. Ceratioidei: Development and relationships. pp. 325–334, In: Moser, H. G., W. J. Richards, D. M. Cohen, M. P. Fahay, A. W. Kendall, Jr., and S. L. Richardson (editors), Ontogeny and Systematics of Fishes, Spec. Publ. No. 1, Amer. Soc. Ichthy. Herpet., ix , 760 pp.
Bertelsen, E., and G. Krefft. 1965. On a rare ceratioid fish, Linophryne lucifer Collett, 1886. Vidensk. Medd. fra Dansk naturh. Foren., 128: 293–301.
Bertelsen, E., and G. Krefft. 1988. The ceratioid family Himantolophidae (Pisces, Lophiiformes). Steenstrupia, 14(2): 9–89.
Bertelsen, E., and T. W. Pietsch. 1975. Results of the research cruises of FRV “Walther Herwig” to South America. XXXVIII. Osteology and relationships of the ceratioid anglerfish genus Spiniphryne (family Oneirodidae). Arch. FischWiss., 26(1): 1–11.
Bertelsen, E., and T. W. Pietsch. 1977. Results of the research cruises of the FRV “Walther Herwig” to South America. XLVII. Ceratioid anglerfishes of the family Oneirodidae collected by the FRV “Walther Herwig.” Arch. FischWiss., 27(3): 171–189.
Bertelsen, E., and T. W. Pietsch. 1996. Revision of the ceratioid anglerfish genus Lasiognathus (Lophiiformes: Thaumatichthyidae), with the description of a new species. Copeia, 1996(2): 401–409.
Bertelsen, E., and T. W. Pietsch. 1998. Revision of the deepsea anglerfish genus Rhynchactis Regan (Lophiiformes: Gigantactinidae), with descriptions of two new species. Copeia, 1998(3): 583–590.
Bertelsen, E., T. W. Pietsch, and R. J. Lavenberg. 1981. Ceratioid anglerfishes of the family Gigantactinidae: Morphology, systematics, and distribution. Nat. Hist. Mus. L. A. Co., Contri. Sci., 332, vi , 74 pp.
Hansen, K., and P. J. Herring. 1977. Dual bioluminescent systems in the anglerfish genus Linophryne (Pisces: Ceratioidea). J. Zool., London, 182: 103–124.
Herring, P. J. 2000. Species abundance, sexual encounter and bioluminescent signaling in the deep sea. Phil. Trans. Roy. Soc. London, B, 355: 1273–1276.
Herring P. J. 2002. Biology of the deep sea. Oxford Univ. Press. 314 pp.
Herring, P. J., and O. Munk. 1994. The escal light gland of the deep-sea anglerfish Haplophryne mollis (Pisces: Ceratioidei), with observations on luminescence control. J. Mar. Biol. Assn. U.K., 74: 747–763.
Herring, P. J., E. A. Widder, and O. Munk. 1994. Flashing anglerfish; an unexpected signalling system. Abstracts of the Eighth Deep Sea Biology Symposium, Monterey, California, 1997: 52.
Hickling, C. F. 1925. A new type of luminescence in fishes. J. Mar. Biol. Assoc. U. K., N. S., 13: 914–937.
Kottelat, M., and C. Vidthayanon. 1993. Boraras micros, a new genus and species of minute freshwater fish from Thailand (Teleostei: Cyprinidae). Ichthy. Explor. Freshwaters, 4(2): 161–176.
Marshall, N. B. 1967a. The olfactory organs of bathypelagic fishes. Symp. Zool. Soc. London, 19:57–70.
Marshall, N. B. 1967b. The organization of deep-sea fishes. pp. 473–479, In: F. M. Bayer, C. P. Idyll, J. I. Jones, F. F. Koczy, A. A. Myrberg, C. R. Robins, F. G. W. Smith, G. L. Vos, E. J. F. Wood, and A. C. Jensen (editors), Proceedings of the International Conference on Tropical Oceanography, 17–24 November 1965, Miami Beach, Florida, Studies in Tropical Oceanography, Miami, Vol. 5.
Munk, O. 1964. The eyes of some ceratioid fishes. Dana Rept., 62, 17 pp.
Munk, O. 1966. Ocular anatomy of some deep-sea teleosts. Dana Rept., 70, 62 pp.
Munk, O. 1988. Glandular tissue of escal light organ in the deep-sea anglerfish Oneirodes eschrichti (Pisces, Ceratioidei). A light and electron microscopic study. Vidensk. Meddr. Dansk Naturh. Foren, 147: 93–120.
Munk, O. 1992. Accessory escal gland (AEG) in some deep-sea anglerfishes. Acta Zool., 73(1): 33–37.
Munk, O. 1998. Light guides of the escal light organs in some deep-sea anglerfishes (Pisces: Ceratioidei). Acta Zool., 79(3): 175–186.
Munk, O. 1999. The escal photophore of ceratioids (Pisces; Ceratioidei)—a review of structure and function. Acta Zool., 80(4): 265–284.
Munk, O. 2000. Histology of the fusion area between the parasitic male and the female in the deep-sea anglerfish Neoceratias spinifer Pappenheim, 1914 (Teleostei, Ceratioidei). Acta Zool., 81(4): 315–324.
Munk, O., and E. Bertelsen. 1980. On the esca light organ and its associated light-guiding structures in the deep-sea anglerfish Chaenophryne draco (Pisces, Ceratioidei). Vidensk. Meddr. Dansk naturh. Foren., 142: 103–129.
Munk, O., and E. Bertelsen. 1983. Histology of the attachment between the parasitic male and the female in the deep-sea anglerfish Haplophryne mollis (Brauer, 1902) (Pisces, Ceratioidei). Vidensk. Meddr. Dansk naturh. Foren., 144: 49–74.
Munk, O., and P. J. Herring. 1996. An early stage in development of escae and caruncles in the deep-sea anglerfish Cryptopsaras couesi (Pisces: Ceratioidei). J. Mar. Biol. Assn. U.K., 76: 517–527.
Munk, O., K. Hansen, and P. J. Herring. 1998. On the development and structure of the escal light organ of some melanocetid deep sea anglerfishes (Pisces: Ceratioidei). J. Mar. Biol. Assn. U.K., 78: 1321–1335.
Parr, A. E. 1927. Scientific results of the Third Oceanographic Expedition of the "Pawnee" 1927. Ceratioidea. Bull. Bingh. Oceanogr. Coll., Yale University, 3(1): 1–34.
Parr, A. E. 1930. On the probable identity, life-history and anatomy of the free-living and attached males of the ceratioid fishes. Copeia, 1930(4): 129–135.
Paxton, J. R. 1998. Squirrelfishes and their allies. pp. 160–164, In: J. R. Paxton and W. N. Eschmeyer (editors), Encyclopedia of Fishes, Second edition, Weldon Owen Pty Limited, McMahons Point, New South Wales, Australia.
Pietsch, T. W. 1974. Osteology and relationships of ceratioid anglerfishes of the family Oneirodidae, with a review of the genus Oneirodes Lütken. Nat. Hist. Mus. L. A. Co., Sci. Bull., 18, 113 pp.
Pietsch, T. W. 1975. Precocious sexual parasitism in the deep-sea ceratioid anglerfish Cryptopsaras couesi Gill. Nature, 256: 38–40.
Pietsch, T. W. 1976. Dimorphism, parasitism and sex: reproductive strategies among deepsea ceratioid anglerfishes. Copeia, 1976(4): 781–793.
Pietsch, T. W. 1979. Ceratioid anglerfishes of the family Caulophrynidae with the description of a new genus and species from the Banda Sea. Contrib. Sci., Nat. Hist. Mus. Los Angeles Co., 310: 1–25.
Pietsch, T. W. 1984. Lophiiformes: Development and relationships. pp. 320–325, In: Moser, H. G., W. J. Richards, D. M. Cohen, M. P. Fahay, A. W. Kendall, Jr., and S. L. Richardson (editors), Ontogeny and Systematics of Fishes, Spec. Publ. No. 1, Amer. Soc. Ichthy. Herpet., ix , 760 pp.
Pietsch, T. W. 1986. Systematics and distribution of bathypelagic anglerfishes of the family Ceratiidae (Order: Lophiiformes). Copeia, 1986(2): 479–493.
Pietsch, T. W. 2005. Dimorphism, parasitism, and sex revisited: modes of reproduction among deep-sea ceratioid anglerfishes (Teleostei: Lophiiformes). Ichthyol. Res., 52: 207–236.
Regan, C. T. 1925a. Dwarfed males parasitic on the females in oceanic angler-fishes (Pediculati, Ceratioidea). Proc. Roy. Soc., B, 97: 386–400.
Regan, C. T. 1925b. New ceratioid fishes from the N. Atlantic, the Caribbean Sea, and the Gulf of Panama, collected by the “Dana.” Ann. Mag. Nat. Hist., Ser. 8, 8(62): 561–567.
Regan, C. T. 1926. The pediculate fishes of the suborder Ceratioidea. Dana Oceanogr. Rept. 2, 45 pp.
Regan, C. T., and E. Trewavas. 1932. Deep-sea anglerfish (Ceratioidea). Dana Rept., 2, 113 pp.
Roberts, T. R. 1986. Danionella translucida, a new genus and species of cyprinid fish from Burma, one of the smallest living vertebrates. Env. Biol. Fish. 16:231–241.
Saemundsson, B. 1922. Zoologiske meddelelser fra Island. XIV. 11 Fiske, ny for Island, og supplerende om andre, tidligere kendte. Vidensk. Medd. Dansk Naturh. Foren., 74: 159–201.
Saruwatari, T., T. W. Pietsch, A. M. Shedlock, I. Oohara, K. Itaya, T. Chiyotani, M. Abe, K. Ishihara, A. Sakai, A. Sugawara, T. Inagaki, K. Ishida, T. Komatsu, T. Sakai, and T. Kobayashi. 2001. Sibling analysis between female and parasitic males of a ceratioid anglerfish (Cryptopsaras couesi, Ceratiidae, Teleostei). DNA Polymorphism, 9: 82–85. [In Japanese.]
Watson, W., and H. J. Walker. 2004. The world’s smallest vertebrate, Schindleria brevipinguis, a new paedomorphic species in the family Schindleriidae (Perciformes: Gobioidei). Rec. Aust. Mus. 56:139–142.
Weitzman, S. H., and R. P. Vari. 1988. Miniaturization in South American freshwater fishes; an overview and discussion. Proc. Biol. Soc. Wash., 101:444–465.
Winterbottom, R., and A. R. Emery. 1981. A new genus and two new species of gobiid fishes (Perciformes) from the Chagos Archipelago, central Indian Ocean. Envir. Biol. Fishes, 6(2): 139–149.
Title Illustrations

| Scientific Name | Ceratioidei |
|---|---|
| Specimen Condition | Dead Specimen |
| Sex | Female |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Theodore W. Pietsch

|
About This Page
Theodore W. Pietsch

University of Washington, Seattle, Washington, USA

University of Washington, Seattle, Washington, USA
Correspondence regarding this page should be directed to Theodore W. Pietsch at and Christopher P. Kenaley at
Page copyright © 2005 Theodore W. Pietsch
 Page: Tree of Life
Ceratioidei. Seadevils, Devilfishes, Deep-sea Anglerfishes.
Authored by
Theodore W. Pietsch and Christopher P. Kenaley.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Ceratioidei. Seadevils, Devilfishes, Deep-sea Anglerfishes.
Authored by
Theodore W. Pietsch and Christopher P. Kenaley.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 02 November 2005
- Content changed 02 October 2007
Citing this page:
Pietsch, Theodore W. and Christopher P. Kenaley. 2007. Ceratioidei. Seadevils, Devilfishes, Deep-sea Anglerfishes. Version 02 October 2007 (under construction). http://tolweb.org/Ceratioidei/22000/2007.10.02 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site