Testudines
Turtles, tortoises and terrapins
Peter A. Meylan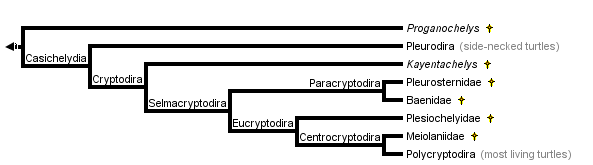

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxTree from Gaffney and Meylan (1988)
Introduction
Several different scientific names are used for turtles including Chelonia, Chelonii, Testudines, and Testudinata. The earliest fossils are from the beginning of the age of dinosaurs, in the late Triassic. The Testudines reached its greatest diversity by the end of the Cretaceous. Today only 260 species representing 13 families survive. Although turtles are abundant in the tropics, they also are quite diverse in temperate regions and have been recorded in Arctic waters.
Records from Olduvai Gorge indicate that men have eaten turtles for at least 2 million years. We have had a severe impact on turtles, causing the extinction of many forms, especially land tortoises. Today the problem is quite serious with many land tortoises, sea turtles and aquatic forms facing extinction. Loss and degradation of habitat and continued killing of reproductive females on the nesting beach and removal of their eggs are the biggest problems. The future of many of these survivors from the age of dinosaurs will depend on a conscious effort on our part.
Turtles (including tortoises and terrapins) are characterized by a shell that completely encloses both of the limb girdles. The shell is composed of a dorsal carapace of dermal bone that incorporates endochondral contributions from the vertebrae and ribs and a ventral plastron of clavicles and interclavicles anteriorly and abdominal ribs posteriorly. No turtles have teeth on their jaws, and all have the external ear supported by a large, semicircular quadrate.


Figure 1- The turtle shell makes all members of this group immediately identifiable. This specimen has been prepared to show that the shoulder girdle and pelvis are enclosed within the shell, a feature that is unique to turtles. Photograph copyright © E. S. Gaffney.
Characteristics
The monophyly of turtles has never been questioned. The following characters are synamorphies for the group:
- All turtles have a bony shell consisting of a carapace formed from costal bones with fused ribs, neural bones with fused thoracic vertebrae, and peripheral bones; a plastron formed from interclavicle, clavicle, and three to five additional pairs of dermal bones sutured together. The carapace and plastron articulate at the lateral margin, enclosing the shoulder girdle and pelvic girdle. It is incorporation of the ribs into the carapace that results in the girdles being enclosed by ribs (see Ruckes, 1929), for developmental studies. It is the incorporation of the ribs into the carapace that results in the girdles being enclosed by ribs (see Ruckes, 1929 for developmental studies.
- In all turtles the quadrate is concave posteriorly and exposed laterally on the cheek. The squamosal is limited to the dorsal half of the cheek, and the quadratojugal and quadrate are relatively large. This is in contrast to the primitive amniote condition in which the quadrate is small and entirely covered laterally by a large squamosal.
- Postparietals are absent so that the post-temporal fenestra is bordered only by parietals and supratemporal in the most primitve turtle Proganochelys (Gaffney 1990).
- The maxilla, premaxilla, and dentary are without teeth but rather covered by a horny triturating surface.
- The stapes is solid and rod-like, without a foramen or process as seen in captorhinids and generalized amniotes (Gaffney 1979).
- The postfrontal is absent, resulting in broad contact between prefrontal and postorbital, and between the frontal and postorbital (Gaffney 1990).
Proganochelys is the most primitive turtle (for an illustration see the Amniotes page). Essentially, all other turtles are placed in the Casichelydia.
Casichelydia
Much of the early evolution of turtles involved a reduction in the number of bones of the skull.
- The generalized amniote condition of a lacrimal bone and lacrimal duct is present in Proganochelys (Gaffney, 1990), but absent in all other turtles.
- More than one vomer (usually a pair) seems to be primitive for all amniotes (Romer, 1956). Proganochelys (Gaffney, 1990) has two, while all other turtles have one.
- The supratemporal bone present in the primitive tetrapods, primitive amniotes, and Proganochelys (Gaffney,1990) is also absent in all other turtles.
In addition to the reduction in the number of elements there is also a closing up of the skull which results in a more solid structure.
- The moveable articulation between the neurocranium and the palatoquadrate elements found in more generalized amniotes (Romer, 1956) is retained in Proganochelys but these units are tightly sutured in all other turtles (Gaffney, 1979).
- Generalized amniotes, such as captorhinids (Heaton, 1979; Gaffney, 1990), have a middle ear region that is open at least laterally and ventrally. Proganochelys also has an open ear region, but in all other turtles there is a variably developed flange of the quadrate that forms at least a partial lateral wall to the middle ear region.
- Proganochelys agrees with the primitive amniote condition in having an opisthotic paroccipital process that is only loosely sutured to the more anterior elements (Gaffney, 1990). In the advanced condition, found in all other turtles, this process is tightly fused along its anterior margin to the quadrate and squamosal (Gaffney, 1979, 1990).
Cryptodira
Most of the diversity of living turtles (10 of 13 families) belong to this group of turtles. All living members can pull their neck inside the shell between the shoulder girdles, hence the name Cryptodira, which means "hidden neck".
- All living cryptodires have the main adductor tendon for the lower jaws directed over a process on the otic chamber formed by the prootic and quadrate (Schumacher, 1973; Gaffney, 1975, 1979), the processus trochlearis octicum. Although the tendon itself is never fossilized, the thickened bone on the anterodorsal surface of the prootic, quadrate, and sometimes the parietal, is preserved in fossil skulls. Proganochelys lacks an otic process and this thickening is absent in all other amniotes (Gaffney, 1990), and this is interpreted as the primitive chelonian condition. Pleurodires have no indication of thickening or of an otic trochlea, rather they have the condition seen in Proganochelys (Gaffney, 1990). Although thickened bone in the prootic and the quadrate per se is a relatively simple feature, the entire system that redirects muscle action is complex. It includes a cartilaginous covering of the process, and a true synovial capsule making up the articular region (all described in Gaffney, 1975, 1979).
- All cryptodirans have a vertical flange on the external process of the pterygoid. Proganochelys has a small pterygoid flange (in turtles termed the processus pterygoidus externus) that has a rolled edge rather than a swollen one (Gaffney 1990). Cryptodires have a vertical plate, oriented anteroposteriorly that bears the cartilage and mundplatte laterally. No other turtles or generalized amniotes have this vertical plate on the processus pterygoidus externus, which is, therefore, interpreted as a crytpodire synapomorphy. In pleurodires, the processus pterygoidus externus is laterally directed and is covered by a curved anterolateral facing plate.
- In cryptodires there is always contact between the prefrontal and vomer. In Proganochelys the ventral process of the prefrontal is extensive but does not reach the vomer (Gaffney 1990). In pleurodires, the prefrontal also does not reach the vomer.
Selmacryptodira
The Selmacryptodira includes all crytpodires other than Kayentchelys aprix from the Jurassic of Arizona.
- Within the cryptodires only Kayentachelys lacks a posteromedial process of the pterygoid that prevents ventral exposure of the prootic and forms the floor of the middle ear. Before the discovery of Kayentachelys (Gaffney et al., 1987), Gaffney (1975) considered this feature a cryptodiran synamorphy. Now it is interpreted as a synamorphy of the Selmacryptodira (Gaffney and Meylan, 1988), the group consisting of all cryptodires except Kayentachelys. This feature does not occur outside of turtles. In pleurodires the prootic is ventrally exposed.
- Among amniotes, the presence of palatal teeth is widespread. Proganochelys has palatal teeth and this is interpreted as the primitive condition. All pleurodires lack palatal teeth and all cryptodires, except Kayentachelys, also lack them. Given the strong evidence that Kayentachelys is a cryptodire, palatal teeth are apparently lost independently in pleurodires and cryptodires.
Paracryptodira
The Paracryptodira includes two, largely Cretaceous families that are now extinct, the Pleurosternidae and Baenidae. The Pleurosternidae is known from North America, Europe, and possibly Asia. The Baenidae is only known from North America. The Paracryptodira includes those cryptodires with a more advanced pattern of blood flow to the head and a simplified plastron.
- In all known members the canalis caroticus internus (internal carotid canal) is partially formed by the pterygoid. In generalized amniotes, the foramen by which the internal carotid artery enters the skull is formed entirely within the basisphenoid. This is also the case in Proganochelys and Kayentachelys (for Pleurodire condition see below) (Gaffney, 1990). In all other cryptodires, an extension of the pterygoid posteriorly and medially forms at least the lateral wall. In pleurosternids and baenids, both the pterygoid and the basisphenoid form the actual entry foramen of the carotid (supporting monophyly of the Paracryptodira), while in other cryptodires the canals are buried within the pterygoid (Gaffney, 1979; Gaffney and Meylan, 1988).
Eucryptodira
- In all members of this group the canalis caroticus is entirely formed by the pterygoid.
- All Eucryptodires also lack a mesoplastra. The complete absence of mesoplastra also characterizes the pleurodiran family Chelidae. The presence of mesoplastra in Proganochelys and most Pleurodires is interpreted as the primitive condition and their absence is derived.
Centrocryptodira
The Centrocryptodira includes those cryptodires with a more advanced condition of the cervical vertebrae.
- The Centrocryptodira get their name from the presence of formed (concave or convex) articulations between succeeding cervical vertebrae. In plesiochelyids the cervical centra are amphicoelus and this is interpreted as the primitive chelonian condition (Gaffney,1990). If the baenid cladogram of Gaffney (1972) and Gaffney and Meylan (1988) is accepted, then the primitive baenids are amphicoelous and advanced ones have formed centra. Formed centra appear independently within a number of other amniote groups, and the widely divergent central articulation pattern in turtle necks (Williams, 1950) is also evidence of homoplasy. In the phylogenetic arrangement used here they would have to appear independently within Pleurodira, Centrocryptodira, and Baenidae.
- A thick floor of the canalis caroticus internus in the pterygoid is also a feature of this group. Within those turtles in which the pterygoid forms the canalis caroticus internus, the plesiochelyids have a thin floor, here interpreted as the primitive condition. In some specimens of Plesiochelys (Gaffney, 1976), the seam or suture closing the canalis is still present. The thick condition lacks indication of a suture and is seen in all other eucryptodires. Presumably the thick, seamless condition is more advanced because during development the canalis floor is thin at first and shows the enclosing of the carotid (Kunkel, 1912).
Fossil Forms
The major groups of turtles already were in existence by the late Triassic, about 210 million years ago. Thus, the origin of turtles must have occurred before this time. The Permian reptile Eunotosaurus in the past was proposed as the ancestor to turtles. This idea has been based on the shell-like structure covering its body which was made up of broadly expanded ribs. However, it has been pointed out recently that the shell of turtles is made up of narrow ribs covered by dermal bone, not broadly expanded ribs. The make-up of the shell and the presence of an ectopterygoid bone in the skull suggest that this genus is not close to the origin of turtles.
Another potential candidate for the closest relatives of turtles identified during the 1970's (Gaffney & Meylan 1988, Gauthier et al. 1988) is the "cotylosaur" family, Captorhinidae. Like all turtles, members of this group lack the ectopterygoid and temporal bones and have a large medial process of the jugal. These lizard-like anapsids show no sign of a shell, and there are still no fossils that show a partly developed turtle shell. However, "parareptile" groups including procolophonids (Laurin & Reisz 1995) and pareiasaurs (Lee 1997) have subsequently been argued to be closer to the origin of turtles than captorhinids. More recently, an old idea, that the origin of turtles actually lies among the diapsids, has received new support from some morphologists (deBraga & Rieppel 1997). Molecular biologists have also provided data that suggest that turtles are diapsids and now consider that the main question is where among the diapsids turtles have their origin (Hedges & Poling 1999, Cao et al. 2000). Go to the Discussion of Phylogenetic Relationships on the Amniota page for more information.
There are many interesting and important turtles that are only known as fossils. The most important of these is the most primitive turtle, Proganochelys. This turtle shows primitive features absent from modern turtles that make it useful as a benchmark for turtle evolution. Equally as old as Proganochelys is the oldest known sideneck, Proterochersis. It has several of the same features of other sidenecks such as the pelvis fused into the shell. Its presence in the late Triassic indicates that the Pleurodire-Cryptodire dichotomy (see phylogeny and classification below) had taken place by this time. The earliest known cryptodire is Kayentachelys from the middle Jurassic of North America. By the late Jurassic marine cryptodires were common in many areas of Europe and Asia. Many of these belong to the extinct family Plesiochelyidae. Two closely related families, the Pleurosternidae and Baenidae, were important groups at the close of the Cretaceous, but both were extinct soon after.
Discussion of Phylogenetic Relationships
Gaffney (1984) provides a complete historical overview of theories of interrelationships among different groups of turtles, from Linnaeus` concept of the single genus Testudo to the more recent views of the last several decades. The starting point for modern discussions of turtle phylogeny is Williams (1950). Although the emphasis of Williams` work was an analysis of variation in the structure of the cervical vertebrae, he provided a complete classification of fossil and living turtles. Since the mid 1970's, Gaffney and coworkers (Gaffney, 1975, 1984, 1996; Gaffney and Meylan, 1988; Gaffney et al., 1991) have challenged much of the phylogenetic arrangement of the major groups proposed by Williams using an extensive morphological data set. Because it is the most complete analysis to date, we follow their arrangement for the turtle pages of the Tree of Life. In addition to the morphological studies, a few papers have examined higher relationships of turtles using nonmorphological data: Chen et al. (1980) used an immunological distance approach, and Bickham and Carr (1983) analyzed variation in chromosome number and morphology among the families of the Cryptodira.
Shaffer, Meylan, and McKnight (1997) published the first study using genetic sequence data to determine the higher relationships among turtles. They studied two genes in 23 living genera of turtles and combined their findings with a new morphological data set. This study supports much of the phylogenetic hypothesis of Gaffney and coworkers. The major differences are in the relationships among the living families of cryptodires (Polycryptodira) and among members of the Chelidae (Pleurodira). See the molecular phylogeny page for more details.
The morphological evidence of Gaffney and coworkers (especially Gaffney and Meylan, 1988; Gaffney et al., 1991) supports the long-held view that two major living groups (often recognized as suborders), the Pleurodira and Cryptodira, are each monophyletic (see Gaffney (1984) for history). Genetic sequence data (Shaffer et al., 1997) strongly supports this dichotomy.
Within the Cryptodira the Jurassic turtle, Kayentachelys aprix, is considered to be the sister group of all other crytodires (Gaffney et al 1989). The Pleurosternidae (Pleurosternon, Glyptops, Mesochelys) is a group of Cretaceous cryptodires that is most likely the sister group of the Baenidae (Gaffney, 1975, 1996). The Baenidae is a distinctive family of Cretaceous to Eocene North American turtles in which the dorsal lappet of the prefrontal is small or absent. The Pleurosternidae and Baenidae form the Paracryptodira which is the sister group of advanced cryptodires. The latter group has been given the name Eucryptodira, which means true cryptodires. All of the "true" cryptodires have the carotid artery hidden within the pterygoid bone.
The Plesiochelyidae is an extinct late Jurassic to early Cretaceous radiation of marine turtles. This is a separate marine radiation from the one to which living sea turtles belong. It constitutes the sister group of all eucryptodires that have formed (not amphicoelus) cervical vertebrae, a group called the Centrocryptodira.
The sister group of all other centrocryptodires is the Meiolaniidae. This is an extinct family (Cretaceous- Pleistocene) of horned turtles found only in South America, and Australia and adjacent islands.
Ecology
All turtles lay eggs. Most bury their eggs in soil, sand or rotting vegetation, but some lay them on the ground in the open. Turtles do not incubate their eggs or attend them in any way, nor do they exhibit any care of the young. The eggs are incubated by environmental heat. The young break free of the egg using an egg tooth or caruncle after some 45 to 90 days of development and fend for themselves from hatching. The primitive condition for turtles appears to be to lay large clutches of round eggs. Snapping turtles, sea turtles, and soft-shells lay dozens to hundreds of round eggs in a single clutch. Certain side-necks, mud and musk turtles, land tortoises and many pond turtles lay fewer eggs per clutch, sometimes only one or two, and many of these turtles lay oblong rather than round eggs. Many turtles are capable of producing more than one clutch of eggs per year, and sea turtles have been known to produce as many as ten clutches in a single year. It has been determined recently that the sex of most species of turtle is determined by environmental factors, that is to say, sex is not determined genetically but rather by such factors as the temperature of incubation.
Turtles are herbivorous, carnivorous and omnivorous. The majority of turtles are omnivorous, but many have highly specialized diets. Certain land tortoises and sea turtles are strict herbivores, and one marine species has the capacity to digest cellulose. Other marine species are specialists on jellyfish (the leatherback) and sponges (the hawksbill). Turtles of several families specialize on mollusks and have broadly expanded jaws for crushing their prey. Others that specialize on swimming prey have developed a vacuum cleaner approach to feeding (snappers, softshells, and some side-necks), using a strong hyoid apparatus to suck prey into their mouths.
Physiology
Turtles belong to the reptilian grade of physiological organization. They are ectothermic and have relatively low metabolic rates. Being ectotherms, their body temperature remains close to the temperature of their environment, and they are entirely reliant on external sources of heat. Many turtles bask in the sun to raise their body temperature to a point where bodily functions can operate optimally. One species, the leatherback, can maintain a body temperature above that of its environment, but how this is achieved is yet to be determined. Most turtles cannot be active during very hot or very cold periods. Therefore, hibernation in winter and aestivation in summer is common for members of this group.
Turtles breathe with lungs located inside of a rigid ribcage. They therefore must use a different mechanism for breathing than most vertebrates. Muscles in the region of the leg pockets act to inflate the lungs, muscles on the surface of the lungs dorsally and ventrally deflate them. Many turtles augment gas exchange at the lungs with gas exchange in the throat or in the cloaca.
In addition to providing protection for the turtle, the shell of at least some species has an important physiological function. It acts as a "calcium bank". Calcium and other cations are taken from the carapace and plastron to buffer the blood during hibernation when metabolic acids are likely to build up. In other species, it appears that in reproductively active females, calcium is removed from the shell and incorporated into eggshells forming around follicles in the oviducts.
References
Bickham, J.W. and J.L. Carr. 1983. Taxonomy and phylogeny of the higher categories of cryptodiran turtles based on a cladistic analysis of chromosomal data. Copeia 4: 918-932.
Cao, Y., M.D. Sorenson, Y. Kumazawa, D.P. Mindell, and M. Hasegawa. 2000. Phylogenetic position of turtles among amniotes: evidence from mitochondrial and nuclear genes. Gene 259: 139-148.
Chen et al. 1980. Evolutionary relationships of turtles suggested by immunological cross-reactivity of albumins. Comp. Biochem. Physiol. 66B: 421-425.
deBraga M. and O. Rieppel. 1997. Reptile phylogeny and the interrelationships of turtles. Zool. J. Linn. Soc. 120: 281-354.
Ernst, C.H. and R.W. Barbour. 1989. Turtles of the world. Smithsonian Institution Press, Washington D.C.
Ernst, C.H., J.E Lovich, and R.W. Barbour. 1994. Turtles of the United States and Canada. Smithsonian Institution Press, Washington D.C.
Gaffney, E.S. 1972. The systematics of the North American family Baenidae (Reptilia Crytodira). Bull. Amer. Nat. Hist. 147(5):241-320.
Gaffney, E.S. 1975. A phylogeny and classification of the higher categories of turtles. Bull. Amer. Mus. Nat. Hist. 155: 387-436.
Gaffney, E.S. 1976. Cranial morphology of the European Jurassic turtles Portlandemys and Plesiochelys. Bull. Amer. Mus. Nat. Hist. 157(6): 65-376.
Gaffney, E.S. 1977. The side-necked turtle family Chelidae: a theory of relationships using shared derived characters. American Museum Novitates 2620:1-28.
Gaffney, E.S. 1979. Comparative cranial morphology of recent and fossil turtles. Bull. Amer. Mus. Nat. Hist. 164 (2): 65-376.
Gaffney, E.S. 1984. Historical analysis of theories of chelonian relationship. Syst. Zool. 33: 283-301.
Gaffney, E.S. 1990. The comparative osteology of the Triassic turtle Proganochelys. Bull. Amer. Mus. Nat. Hist. 194: 1-263.
Gaffney et al. 1987. Modern turtle origins: The oldest known cryptodire. Science 237: 289-291.
Gaffney et al. 1991. A computer assisted analysis of the relationships of the higher categories of turtles. Cladistics: 313-335.
Gaffney, E.S. 1996. The postcranial morphology of Meiolania platyceps and a review of the Meiolaniidae. Bull. Am. Mus. Nat. Hist. 229:1-166.
Gaffney, E.S. and P.A. Meylan. 1988. A phylogeny of turtles. pp. 157-219 in M.J. Benton (ed.) The Phylogeny and Classification of the Tetrapods, Vol.1, Clarendon Press, Oxford, 1988.
Gauthier, J., A.G. Kluge, and T. Rowe. 1988. Amniote phylogeny and the importance of fossils. Cladistics 4: 105-209.
Gibbons, J.H. 1990. Life history and ecology of the slider turtle. Smithsonian Institution Press, Washington D.C.
Harless, M. and H. Morelock, 1979. Turtles: Perspective and Research. Wiley, New York.
Heaton, M.J. 1979. Cranial anatomy of primitive captorhinid reptiles from the late Pennsylvanian and Early Permian Oklahoma and Texas. Oklahoma Geol. Surv. Bull. 127: 1-84.
Hedges, S.B. and L.L. Poling. 1999. A molecular phylogeny of reptiles. Science 283: 998-1001.
Iverson, J.B. 1992. A revised checklist with distribution maps of the turtles of the world. Earlham College (privately printed), Richmond IN.
Kunkel, B.W. 1912. The development of the skull of Emys lutaria. J. Morph. 23: 693-780.
Laurin, M. and R.R. Reisz. 1995. A reevaluation of early amniote phylogeny. Zool. J. Linn. Soc. 113: 165-223.
Lee, M.S.Y. 1997. Pareiasaur phylogeny and the origin of turtles. Zool. J. Linn. Soc. 120:197-280.
Meylan, P.A and E.S. Gaffney. 1989. The skeletal morphology of the Cretaceous Crytodiran turtle, Adocus, and the relationships of the trionychoidea. Amer. Mus. Nov. 2941: 1-60.
Pritchard, P.C.H. 1979. Taxonomy, evolution, and zoogeography. In: M. Hareless and H. Morelock (eds) Turtles, perspectives and research. Wiley, New York, p.1-42.
Pritchard, P.C.H. 1979. Encyclopedia of Turtles. T. F H. Publications, Neptune, N.J.
Romer, A.S. 1956. Osteology of the Reptiles. Univ. of Chicago Press.
Ruckes, H. 1929. Studies in chelonian osteology. Part II. The morpoholgical relationships between the girdles, ribs, and carapace. Ann. N.Y. Acad. Sci. 31, 81-120.
Schumacher, G.H. 1973. The head muscles and hyolaryngeal skeleton of turtles and crocodilians. In: C.Gans and T.S.Parsons (eds) Biology of the Reptilia, Vol.4. Academic Press, London, New York, p 101-199.
Shaffer, H.B., P. Meylan, and M.L. McKnight. 1997. Tests of turtle phylogeny: Molecular, morphological, and paleontological approaches. Systematic Biology 46:235-268.
Williams, E.E. 1950. Variation and selection in the cervical central articulations of living turtles. Bull. Amer. Mus. Nat. Hist. 94: 505-562.
Information on the Internet
- The UC Museum of Paleontology also has phylogenetic information concerning Testudines, as well as a listing of other Internet sites- Internet Turtle Resources.
- Order Testudines. The University of Michigan Museum of Zoology Animal Diversity Web.
- Phylogeny of Turtles. Eugene S. Gaffney.
- Chelonian Research Foundation publishes the Turtle and Tortoise Newsletter.
- Turtles of the World. A prototype for a global database of turtle and tortoise distribution.
- Crocodilian, Tuatara and Turtle Species of the World. An online taxonomic and geographic reference.
- Turtle Photographic Collection
Title Illustrations

| Scientific Name | Lepidochelys olivacea |
|---|---|
| Location | Nancite Beach, Costa Rica |
| Comments | Olive ridley turtle |
| Specimen Condition | Live Specimen |
| Copyright |
© 1996 Greg and Marybeth Dimijian

|
About This Page
I have relied heavily on the work of my esteemed colleage, Gene Gaffney, in the preparation of these pages. Eric Ganko was instrumental in preparing a preliminary draft.
Peter A. Meylan

Eckerd College NAS, St. Petersburg, Florida, USA
Correspondence regarding this page should be directed to Peter A. Meylan at
Page copyright © 2012
All Rights Reserved.
- First online 31 May 2001
Citing this page:
Meylan, Peter A. 2001. Testudines. Turtles, tortoises and terrapins. Version 31 May 2001. http://tolweb.org/Testudines/14861/2001.05.31 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site