Ommastrephes
Ommastrephes bartramii
Red squid
Richard E. Young and Michael VecchioneIntroduction
Ommastrephes bartramii, commonly known as akaika, red squid, red ocean squid, red flying squid, flying squid, neon flying squid and Bartram's squid (among many other names), is the most broadly distributed species in the family Ommastrephidae with a circumglobal subtropical to temperate distribution (Murata, 1990).
In the North Pacific, O. bartramii is especially abundant and commercial catches have reached over 300,000 mt/year. During summer and fall in the North Pacific, O. bartramii is fished primarily between 36° and 46°N latitude, and in winter most squid are thought to migrate south to the subtropics between about 25° and 35°N latitude where spawning occurs (Yatsu, et al., 1998; Ichii, et al., in press).
Brief diagnosis:
An ommastrephin with ...
- silver band on ventral midline of mantle.
- 4-7 toothed suckers on club proximal to most proximal carpal sucker.
Characteristics
- Arms
- Arms tips in subadults not unusually attenuate; arms I with 22-27 pairs of suckers (Wormuth, 1976).
- Hectocotylus (Wormuth, 1976)
- Right or left arm IV hectocotylized.
- Ventral sucker series with 9-27 suckers; dorsal series with 10-25 suckers. Size and dentition normal.
- Lateral membrane absent.
- Hectocotylus without pores.
- Width of ventral protective membrane of arm III much greater than arm width.
- Tentacles
- Largest club suckers with small pointed teeth and one large pointed tooth in each quadrant.
- Club with 4 - 7 toothed club suckers proximal to most proximal carpal sucker. This character (along with the absence of a dorsal mantle photophore patch) is especially useful in separating O. bartramii from S. pteropus in the Atlantic Ocean.
- Two rarely three carpal knobs present (Sasaki, 1929).
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window
Figure. Oral-oblique view of a sucker ring from a large sucker of the medial series on the manus of O. bartramii. Note the large tooth in each quadrant. Photograph by R. Young.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window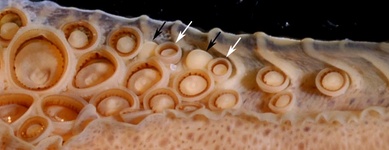
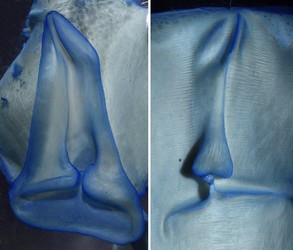
Figure. Oral view of the proximal region of the tentacular club of O. bartramii, 145 mm ML, Hawaiian waters. The arrows indicate the carpal locking-apparatus: white arrows - smoothed ringed locking suckers; black arrows - locking knobs. Note the four normal suckers proximal to the locking apparatus. Photographs by R. Young.
- Largest club suckers with small pointed teeth and one large pointed tooth in each quadrant.
- Head
- Beaks: Descriptions can be found here: Lower beak; upper beak.
- Beaks: Descriptions can be found here: Lower beak; upper beak.
- Funnel/mantle locking-apparatus
- Mantle component of locking-apparatus with anterior bifurcation.
- Mantle and funnel components of locking-apparatus not fused.
- Mantle component of locking-apparatus with anterior bifurcation.
- Mantle
- Unique, elongate silvery band on mid-ventral surface of mantle.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window

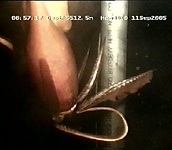
Figure. Ventral views of O. bartramii showing midventral strip. Top - Chromatophores contracted showing the distinct silvery nature of the ventral mantle strip. Bottom left - Chromatophores cover midventral strip emphasizing strip's presence. Unfortunately this diagnostic character is not always easily seen. Photographs by R. Young. Bottom right - In situ video grab at 512 m depth next to a drilling pipe showing the covered midventral strip and the large protective membrane of arm III. Photograph provided by A.R. Gates, SERPENT project.
- Small, very irregular, subcutaneous photophores on ventral surfaces of mantle, head and each arm IV.
Comments
More details of the description of O. bartramii can be found here.
Life History
Egg masses for O. bartramii have never been observed. Eggs measure about 0.9 x 1.1 mm in size (Sakurai, et al., 1995). Spawning in the North Pacific occurs virtually all year long (Yatsu et al., 1998). Hatchlings measure about 1.1 mm ML (Yatsu and Mori, 2000).
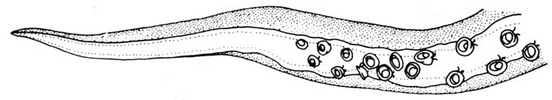
The paralarvae of O.bartramii are well known (Young and Hirota, 1990). Paralarvae are easily identified when they reach about 3 mm ML as the two most useful characters in identifying this species are apparent: The large lateral suckers at the tip of the proboscis and the presence of two chromatophores located near the anteroventral margin of the mantle (see arrows in drawing below). The paralarva at this size also generally has 1 or 2 posteriovental chromatophores. The first juvenile stage is though to be indicated by the separation of the tentacles from one another. At the time of separation, the tentacles are very small compared to the arms.

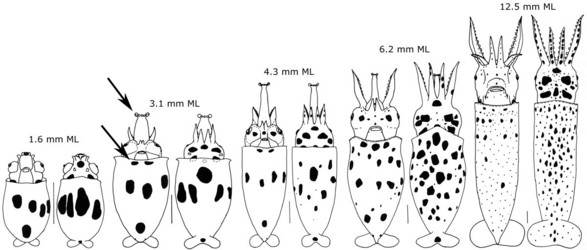
Figure. Paralarval and older stages of O. bartramii. The scale bar is 1 mm. Drawings from Young and Hirota (1990).
Paralarvae have been captured over a broad stretch of the North Pacific from 140°E and 130°W between 25°N and 35°N (Yatsu, et al., 1998) and as far south as 20°N in Hawaiian waters (Young et al., 2000). Paralarvae appear to occur mostly in the upper 25 m during the day and night (Young and Hirota, 1990; Saito and Kubodera, 1993). In the North Pacific paralarvae have been captured over a broad stretch of from 140°E and 130°W between 25° and 35°N (Yatsu, et al., 1998). In the Hawaiian Archipelago, paralarvae are caught where sea surface temperatures were 21°-24°C (Bower, 1994).
Yatsu and Mori (2000) examined paralarval growth rates based on statolith increment counts and they were able to include hatchlings grown from artificial fertilization. The growth curve they determined is ML=1.139e0.063x which gives a size of about 7 mm at 30 days of age. They note proboscis separation was reported by Wormuth et al. (1992) to occur at 7 mm ML and that this size appears to coincide with a change from exponential to linear growth and suggest that feeding habits must change at this point with the presence of functional tentacles. After separation, however, the tentacles are undeveloped and very small (L. Shea, personal communication, see also the Eucleoteuthis page) indicating that considerable growth of the tentacles is necessary before they can participate in feeding although growth of the tentacles is probably rapid.

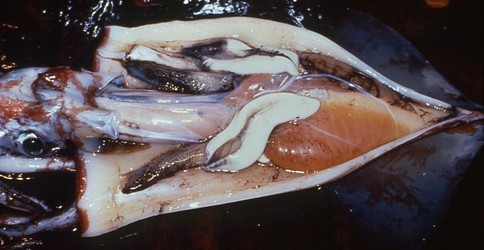
Figure. Ventral view of the opened mantle cavity of O. bartramii, mature female, North Pacific, FTS HOKUSEI MARU. The large nidamental glands are ivory white, the oviducts, packed with mature eggs, are orange and these overlie the off-white colored ovary. Photograph by Jed Hirota.
Maximum size reported by Yatsu (2003) for North Pacific O. bartramii is 45 cm ML (mantle length) for males and 60 cm ML for females. Although females grow to a much larger size than males, the mantle-length - total-weight relationships are indistinguishable and are given by the formula: W= (1.2799 x 10-5)L3.1437 with L (mantle length) in mm and W (weight) in g (Murata, 1990). Most males larger than 30 cm ML are mature and most females larger than 50 cm ML are mature (Yatsu et al., 1998). The life span of O. bartramii is estimated to be about one year (Yatsu, 1997).
Discharged spermatophores, spermatangia, are attached to the oral surface of the buccal membrane. Within the buccal membrane are numerous, small pouches, the spermathecae, which store the sperm from the spermatangia. In Dosidicus gigas, sperm, apparently, swim along the buccal membrane on pathways of seminal fluid that extend from the free end of the spermatangia to the openings of the seminal receptacles (Fernández-Álvarez, et al., 2017).

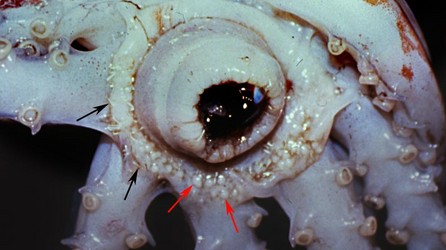
Figure. Oral view of the buccal membrane of O. bartramii, mature female, showing numerous spermatangia (black arrows) on the membrane and adjacent areas, and the bulbous spermathecae (red arrows). Photograph by J. Hirota.
Female O. bartramii probably have continuous asynchronous ovulation with spawning over an extended period with intermittent spawning events (i.e., as the oviducts fill, they are emptied) and somatic growth probably occurs during the period of spawning (Young, et al., 1997). However, the evidence for this strategy is not conclusive. The number of eggs (ova) in both oviducts of a female is known to reach at least 1.4x106 eggs in a female of 590 mm ML and 1.3x106 in a 545 mm ML female (Young et al., 1997).
Biology
Horizontal migration
The population of O. bartramii in the North Pacific annually makes a round-trip migration between its subtropical spawning grounds and its northern feeding grounds near the Subarctic Boundary (Bower and Ishii, 2005). The population consists of two seasonal cohorts, an autumn spawning cohort and a winter-spring spawning cohort. The autumn cohort is rare in the western North Pacific but the winter-spring cohort is found from the western to the eastern North Pacific (Ichii et al., 2009).


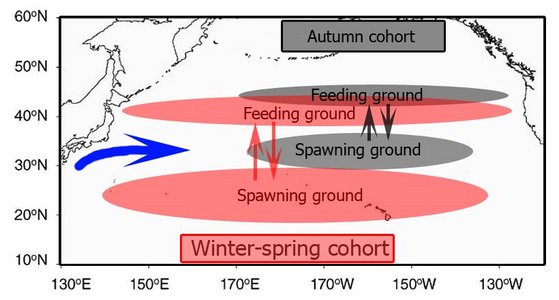
Figure. Map showing the different seasonal positions of the feeding and spawning grounds for the two cohorts of O. bartramii. The blue arrow represents the autumn position and direction of the Kuroshio extension Current during Autumn. Drawing modified from Ichii, et al. (2009).
The autumn cohort grows faster (see duration thin and dashed lines in chart below))during the first half of its life cycle but the winter-spring cohort grows faster during the second half; males and females of the autumn cohort follow separate migration patterns but both sexes of the winter-spring cohort follow nearly identical patterns. These differences result from the seasonal north-south movements of the optimum spawning zone defined by sea surface temperatures (21° - 25°C), and the food-rich zone defined by the transition zone chlorophyll front (Ichii et al., 2009).

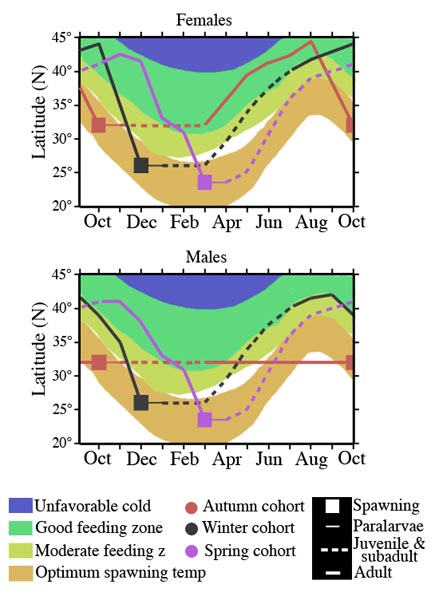
Figure. Migration paths of the cohorts of O. bartramii relative to the changing positions of the spawning and feeding grounds during the year. For clarity, the winter-spring cohort is represented by two cohorts (winter cohort and spring cohort). Note the non- migration of males of the Autumn cohort. The chart, for simplicity (and data limitations), shows rather discrete spawning periods. However, the Autumn cohort hatches mainly from Sept to Dec. and the Winter-spring cohort mainly from November to May. Chart modified from Ichii et al., (2009).
The more northern spawning of the autumn cohort results in juveniles and subadults being in moderate to good feeding environments, resulting in rapid growth while those of the winter-spring cohort don't encounter good feeding conditions until later in life. The males of the autumn cohort become adults before the good-feeding grounds move further north with the changing seasons. As a result the males do not need to follow the feeding grounds northward. Females of O. bartramii, attain a much larger size as adults than males and, therefore, autumn-cohort females follow the feeding grounds northward leaving the males behind. Males of the winter-spring cohort migrate northward with the females until they reach about 25 cm ML. They then separate from females which continue to migrate north and remain in the southern part of the feeding grounds before migrating south a few months earlier than the females. (Ichii et al., 2009)
During autumn the strong Kuroshio Extension Current, which flows from about 140° E. Long. to 170°E over 5 mo., coincides with the optimum spawning spawning temperature. As a result any squid spawned in the Western North Pacific during autumn would be transported to central North Pacific waters by the time they would start migrating northward, thereby removing the cohort from the Western North Pacific. (Ichii et al., 2009)
The life-history migration patterns presented by Ichii et al. (2009) are not fully documented but the evidence supporting their conclusions is very strong.
The total catch of O. bartramii in the North Pacific peaked at 378,000 mt prior to the moratorium on drift net fishing at the end of 1992 (Murata and Nakamura, 1998). The jigging fishery which replaced the drift net fishery now catches between 100,000-200,000 tons/year Ichii et al., 2009).


Figure. Japanese commercial fishing vessel with catch O. bartramii taken by drift net when this fishing method was legal. Photograph by Mike Seki.
Swimming speed
Average swimming speeds have been measured using ultrasonic transmitters attached to free-swimming squid. For squids apparently on the spawning grounds (26°-28°N), swimming speeds ranged between 19 and 25 cm/s (three squid, 44-46 cm ML, for 9 - 36 hrs.) (Nakamura, 1993). Three females (38-42 cm ML) that swam consistently in a southeasterly direction from about 35°N and were apparently migrating had average speeds of 25-30 cm/sec or 54-74% of their ML (Yoshida et al. (1990). Bower and Ishii (2005) noted average speeds up to 49 cm/sec have been recorded.
A tag and release study reported 11 migrating squids (unknown size) that had been released in May-June and recaptured mostly between 1 and 3 months after release (Murata and Hayase (1993). These squid traveled in various directions between NNW and ENE at a combined average speed of 11.8 km/day (14 cm/sec) but two traveled for an average distance of 594 miles at an average speed of 23.1 km/day (27 cm/sec).
Distribution
Vertical Distribution
Judging from the methods used in commercial fisheries [jigging-usually upper 50 m, driftnets-upper 10 m] O. bartramii commonly occupies depths of 0 to 50 m at night at the subarctic boundary in the North Pacific (Murata, 1990). At 37°N latitude (160°E - 172°W) small (about 140-180 mm ML) O. bartramii, several hundred at a time, have been seen gliding above the surface of the water during nighttime and daytime suggesting the habitat for squid in this size range is in near-surface waters during the day and night (Murata, 1988).
A few squid have been tracked by ultrasonic telemetry. In the region of the Pacific Subartic Frontal Zone (ca. 42°-45°N), at night squid swam at depths of 0 - 40 m within the mixed layer (Murata and Nakamura (1998). During the day the two longest records found that one squid swam at depths of 150-200 m during the daytime (10°-6°C) and the other at depths of 160-300 m during the daytime (7°-4°C). A few squid stayed near the surface during the daytime.
In subtropical waters of the North Pacific (ca. 26°-28°N), one acoustically tracked squid stayed at depths between 400 and 700 m in the day (9°C at 600 m) and mostly at 40-70 m at night with occasional excursions to the surface Nakamura (1993). A second squid spent the day at depths greater than 680m (limit of the transmitter) and at depths mostly between 50 and 70 m at night. The greater daytime swimming depths of squid in subtropical waters presumably is related to the greater clarity of these waters, which requires that the squid swim much deeper to reach the same light levels (Murata and Nakamura, 1998).
Presumably O. bartramii can on occasion descend to much greater depths than recorded above. Clarke (1966) reported that O. bartramii in the North Atlantic had tentacles caught in reversing thermometers as deep as 1490 m. Submersible observations in the North Atlantic found O. bartramii at 540-1,050 by day and mostly in surface waters at night with a few individuals to 300m depth; in the South Atlantic O. bartramii was found at 530-950 m, but mostly at 750-850 m by day and near the surface at night (squid ranged from 15-45 cm ML) (Moiseev, 1991).
Geographical Distribution
Cosmopolitan in subtropical and temperate waters. Russian researchers (Dunning, 1998) consider the North Pacific and North Atlantic populations to represent separate subspecies and the southern hemisphere populations to represent a third subspecies. The southern-hemisphere population, however, is discontinuous at the tip of South America and at the southeastern tip of Australia which suggests to Dunning (1998) that the South Pacific population is reproductively separate from the South Atlantic-Indian Ocean population. The northern limit of the population in the South Pacific is approximately 25°N latitude (Dunning, 1998).
References
Bower, J. R. 1994.Distribution of paralarvae of the squid Ommastrephes bartramii near the Hawaiian Archipelago. MS Thesis, Univ. Hawaii, 120 pp.
Clarke, M. R. 1966. A Review of the Systematics and Ecology of Oceanic Squids. Advances in Marine Biology, 4: 91-300.
Dunning, M. C. 1998. A review of the systematics, disturbution, and biology of the arrow squid genera Ommastrephes Orbigny, 1835, Sthenoteuthis Verrill, 1880, and Ornithoteuthis Okada, 1927 (Cephalopoda: Ommastrephidae) . Smithsonian Contributions to Zoology, No. 586: 425-433.
Fernández-Álvarez, F. Á., R. Villanueva, H.-J. T. Hoving, W. F. Gilly. 2017. The fourney of squid sperm. Rev. Fish. Biol. Fisheries. DOI 10.1007/s11160-017-9498-6.
Ichii, I., K. Mahapatra, M. Sakai and Y. Okada. 2009. Life history of the neon flyng squid: effect of the oceanographic regime in the North Pacific Ocean. Mar Ecol Prog Ser. 378: 1-11.
Moiseev, S. I. 1991. Observation of the vertical distribution and behavior of nektonic squids using manned submersibles. Bull. Mar. Sci., 49: 446-456.
Murata, M. 1988. On the flying behavior of neon flying squid Ommastrephes bartrami observed in the central and northwestern North Pacific. Nippon Suisan Gakkaishi, 54: 1167-1174.
Murata, M. 1990. Oceanic resources of squids. Mar. Behav. Physiol., 18: 19-71.
Murata, M. and Y. Nakamura. 1998. Seasonal migration and diel vertical migration of the neon flying squid, Ommastrephes bartramii, in the North Pacific. Contributed papers to the international symposium on large pelagic squids. Japan Marine Fishery Resources Research Center, p. 13-30.
Nakamura, Y. 1993. Vertical and horizontal movements of mature females of Ommastrephes bartramii observed by ultrasonic telemetry. pp. 331-336. In: Okutani, T., R. K. O'Dor and T. Kubodera (eds.) Recent Advances in Fisheries Biology. Tokai Univ Press, Tokyo.
Saito, H. and T. Kubodera. 1993. Distribution of ommastrephid rhynchoteuthion paralarvae (Mollusca: Cephalopoda) in the Kuroshio Region. pp. 457-466. In: Okutani, T., R. K. O'Dor and T. Kubodera (eds.) Recent Advances in Fisheries Biology. Tokai Univ Press, Tokyo.
Sakurai, Y., R. E. Young, J. Hirota, K. Mangold, M. Vecchoine, M. R. Clarke and J. Bower. 1995. Artificial fertilization and development through hatching in the oceanic squids Ommastrephes bartramii and Sthenoteuthis ouananiensis (Cephalopoda: Ommastrephidae). The Veliger, 38: 185-191.
Wormuth, J. H. 1976. The biogeography and numerical taxonomy of the oegopsid squid family Ommastrephidae in the Pacific Ocean. Bull. Scripps Inst. Oceanogr., 23, 90 pp.
Wormuth, J. H., R. K. O'Dor, N. Balch, M. C. Dunning, E. C. Forch, R. F. Harman and T. W. Rowell. 1992. Family Ommastrephidae Steenstrup,1857. Smithson. Contr. Zool., No. 513:105-119.
Yatsu, A., Midorikawa, S., Shimada, T., Uozumi, Y., 1997. Age and growth of the neon flying squid, Ommastrephes bartrami, in the North Pacific Ocean. Fish. Res. 29, 257–270.
Yatsu, H. and J. Mori. 2000. Early growth of the autumn cohort of neon flying squid, Ommastrephes bartramii, in the north Pacific Ocean. Fisheries Research, 45: 189-194.
Yatsu, A., H. Tanaka and J. Mori. 1998. Population structure of the neon flying squid, Ommastrephes bartramii, in the north Pacific Ocean. Contributed papers to the international symposium on large pelagic squids. Japan Marine Fishery Resources Research Center, p. 31-48.
Young, R. E. and J. Hirota. 1990. Description of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) paralarvae with evidence for spawning in Hawaiian waters. Pac. Sci., 44: 71-80.
Young, R. E., Hirota, J. and M. Parry. 1997. Aspects of the ecology of the red squid Ommastrephes bartramii, a potential target for a major Hawaiian fishery. FY1997 progress report. Pelagic Fisheries Research Program, JIMAR, Univ. of Hawaii.
Young, R. E., Hirota, J. and M. Parry. 2000. Aspects of the ecology of the red squid Ommastrephes bartramii, a potential target for a major Hawaiian fishery. FY2000 progress report. 7pp. Pelagic Fisheries Research Program, JIMAR, Univ. of Hawaii.
Title Illustrations

| Scientific Name | Ommastrephes bartramii |
|---|---|
| Location | Northern Hawaiian waters |
| Comments | Photographed in a ship-board aquarium. |
| Specimen Condition | Live Specimen |
| View | Side |
| Size | Estimate of 30-40 cm ML |
| Copyright | © Marc Hughes |
| Scientific Name | Ommastrephes bartramii |
|---|---|
| Location | Off Southern California |
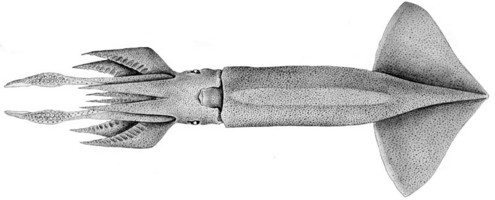
| Reference | Young, R. E. 1972. The systematics and areal distribution of pelagic cephalopods from the seas off Southern California. Smithson. Contr. Zool., 97: 1-159. |
| View | Ventral |
| Size | 211 mm ML |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
©

|
About This Page

University of Hawaii, Honolulu, HI, USA

National Museum of Natural History, Washington, D. C. , USA
Page copyright © 2018 and
 Page: Tree of Life
Ommastrephes . Ommastrephes bartramii . Red squid.
Authored by
Richard E. Young and Michael Vecchione.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Ommastrephes . Ommastrephes bartramii . Red squid.
Authored by
Richard E. Young and Michael Vecchione.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 29 November 2009
- Content changed 20 February 2018
Citing this page:
Young, Richard E. and Michael Vecchione. 2018. Ommastrephes . Ommastrephes bartramii . Red squid. Version 20 February 2018 (under construction). http://tolweb.org/Ommastrephes_bartramii/19947/2018.02.20 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site