Decapodiformes
Squids, cuttlefishes and their relatives
Richard E. Young, Michael Vecchione, and Katharina M. Mangold (1922-2003)The Decapodiformes contains about 95 genera and 450 species in 31 families. Many of these families seem to form natural groups and these are included below without formal names as the validity of the groups has not yet been demonstrated by cladistic methods.



This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The most distinctive feature of decapodiforms is the modification of the fourth pair of arms into tentacles (see title photographs). Since tentacles are known only in the Decapodiformes, determining the primitive form of the tentacle
In some species these are long slender structures that are several times the length of the body and probably function much like a fishing line. In some others, they are short, muscular structures that can be quickly extended (literally shot forward) to grasp prey then completely retracted into pockets in the head.
The Decapodiformes includes species with a wide range of body forms and habits. Muscular sepioids bury in the sand during the day; gelatinous squids swim via slow jet propulsion in the deep-sea while in the same habitat some squids with weak mantle muscles use large muscular fins for swimming; surface-dwelling squid with powerful jet propulsion may undertake migrations of a thousand miles and, when disturbed, dart from the water and glide over the ocean surface like a flying fish. In size they range from Idiosepius with a mantle length of 8 mm at maturity to the giant squid Architeuthis which reaches nearly 18 m in total length. Cephalopods are primarily visual animals even though they possess lateral line analogs, very low frequency hearing and olfaction. The dominance of the visual system is particularly true of decapods. For example, the eye diameter of the small Iridoteuthis is over 40% of the mantle length and large squids have eyes that surpass the eye-size of all other animals big or small. Among decapods, the oegopsid squids characteristically produce small pelagic eggs and the paralarvae develop in the near-surface plankton whereas myopsids and sepioids have benthic eggs that often produce large demersal hatchlings.
The suckers of all decapods have horny rings. These rings often carry sharp claw-like teeth and in some species these have been modified into hooks. The advantages of hooks are uncertain. They could be designed for sinking into soft-bodied prey, such as other squid, or they could act as grappling hooks for hard-body spiny prey where suckers would be ineffective (Young, et al., 1999).
Characteristics
- Arms/tentacles
- fourth arms,of the original 5 arm pairs, modified as tentacles (see title photographs).
- Suckers bilaterally symmetrical.
- Suckers with horny rings; inner rings solid, outer rings composed of many separate pieces.
- Suckers stalks with constricted necks.
 Click on an image to view larger version & data in a new window
Click on an image to view larger version & data in a new window

Figure. Arm suckers. Left - Oral view of arm suckers of Sthenoteuthis oualaniensis, preserved. The reddish, toothed portion is the inner horny ring; the yellow surrounding slats form the outer horny ring. Right - Lateral view of an arm sucker and stalk of Histioteuthis hoylei, preserved. The skin has been removed from the sucker stalk to show its shape: a broad base tapering to a constricted neck where the stalk attaches to the sucker. Photographs by R. Young.
- Arms IV without connecting web.
- Buccal crown
- Buccal crown present.
- Head
- Statocyst with single capsule.
- Superior and posterior buccal lobes widely separated.
- Inferior frontal lobe system of brain absent.
- Photosensitive vesicles
- Photosensitive vesicles lie within cephalic cartilage.
- Photosensitive vesicles lie within cephalic cartilage.
- Fins
- Fins present but without cartilagenous core.
- Fin cartilage present at base of fins often as a plate.
- Shell
- Shell a phragmocone, gladius or absent.
- Shell a phragmocone, gladius or absent.
- Viscera
- Nidamental glands present.
- Crop absent.
- Oviducal glands bilaterally symmetrical.
- Digestive gland duct appendages surrounded by nephridial coelom.
- Nephridial coeloms joined to form a single coelom.
- Visceropericardial coelom extensive.
- Interstellate nerve often present.
Comments
Comparisons of the major taxa within the Decapodiformes
| Shell type | Cornea present | Branchial canal present | Buccal supports with suckers | Tentacle with carpal locking apparatus | Suckers with circulis muscles1 | Tentacle pocket large, tentacles contractile and retractile2 | Fins attach mostly to mantle sides rather than each other and/or shell | Secondary eyelid present | Mantle component of funnel-mantle lock reaches about anterior mantle margin | Habitat | |
| Oegopsida | Gladius | No | Yes | No | Yes/some exceptions | No | No, few exceptions | No, few exceptions | No | Yes, few exceptions | Pelagos |
| Bathyteuthoidea | Gladius | No | Yes | Yes | No | No | Yes | Yes3 | No | Yes | Pelagos |
| Myopsida | Gladius | Yes | Yes | Yes/No | No | Yes | Yes | Yes | No | Yes | Pelagos |
| Idiosepiidae | Gladius | Yes | No | No | No | No | Yes | Yes | No | No | Benthos |
| Spirulidae | Coiled calcified fragmocone | No | No | No | No | No | Yes | No | No | Yes | Pelagos |
| Sepiiidae | Flattened calcified fragmocone | Yes | No | Yes/No | No | Yes | Yes | Yes | yes | No | Benthos |
| Sepiolida | Gladius or absent | Yes | No | No | No | Yes | Yes | Yes | Yes, rare exceptions | No, rare exceptions | Benthos |
1. Readily visible in Sepiolida; others require sectioning. 2. All tentacles are contractile; retractile tentacles can be coiled or partly coiled which requires a larger tentacle pocket. 3. Difficult to determine in Bathyteuthis.
Discussion of Phylogenetic Relationships
At present we recognize four major lineages within the Decapodiformes but the relationships between them are unresolved. Two taxa, Bathyteuthoidea and Idiosepiidae, which have subordinal rankings are included here as their ordinal relationships are uncertain. The Bathyteuthoidea shows relationships with both the Oegopsida and the Myopsida while the Idiosepiidae shows relationships with the Myopsida and the Sepioidea. Among the orders, the Myopsida shows relationships with both the Oegopsida and the Sepioidea and the Sepioidea and the Spirulida show relationships.
Since tentacles are known only in the Decapodiformes, there is no "outgroup" that can provide evidence of the primitive form of the tentacular club. The characteristics of the tentacle club, however, are primary features in the characterization of many families within the superorder and understanding their evolutionary pathways is important in eventually unraveling the phylogenetic relationships of these taxa. THEREFORE, AN UNDERSTANDING OF THE STRUCTURE OF THE TENTACLE CLUBS OF THE BASAL TAXA IS IMPORTANT AND CAN BE FOUND HERE.
Among the latter two orders, the closeness of the relationship is uncertain. The Sepiidae of the Sepioidea and the Spirulidae of the Spirulida have substantial fossil records and these records argue for a close relationship between these two orders. In both cases, the presence of an internal calcareous shell with a phragmocone, however, represents the retention of an ancient and plesiomorphic character state, and does not indicate that the animal is an early derivative of the decapodiform clade or close relationships. Some details of the shell structure support a close relationship while others argue against it.
Spirula spirula, the only living member of the Spirulida, is a peculiar cephalopod that has stimulated much discussion regarding its phylogenic position. While the relationships are still unresolved, Young, et al. (1998) support its close relationship to the Sepiidae and other sepioid families based on arguments mostly put forward by Naef (1922) (see drawing below).

Figure. Drawings from Young et al. (1998) modified from Naef (1922) show the similarities between Spirulirostra, Spirulirostrina (fossil relatives of Spirula) and Belosaepia (a fossil relative of Sepia) and Sepia. Note the gray-colored "guard-like sheath" on all species illustrated (it forms the rostrum, shield,etc. on the Sepia cuttlebone). The cuttlebone of Belosaepia lacks the cross-struts of the Sepia cuttlebone and has distinct septa on the ventral side of the siphuncle. The siphuncle is also more ventrally curved than in Sepia. The presumed attachment of the mantle muscle (red) to the ventral process of the sheath is shown in Spirulirostra and Spirulirostrina. The mantle attaches similarly to the recurved ventral process of the cuttlebones.
Both the spirulid and sepiid shells have a distinctive ventral curvature of the apical end and the asymmetrical elaboration of the sheath that includes such similarities as a rostrum and a ventral process (Young, et al., 1998).
Extinct clades
A large number of fossil species possessed a gladius but lacked a phragmocone. They have been included by various authors in the Decapodiformes and/or the Vampyromorpha. Resolution of their relationships is badly needed (Young, et al., 1999).
References
Clarke, M. R. 1988. Evolution of recent cephalopods -- A brief review. P. 331-313. In: Clarke, M. R. and E. R. Trueman (Eds.). The Mollusca. Vol. 12. Paleontology and Neontology of Cephalopods. Academic Press, New York. 355pp.
Herring, P. J., P. N. Dilly and C. Cope. 1985. The photophore morphology of Selenoteuthis scintillans Voss and other lycoteuthids. J. Zool. Lond. 206: 567-589.
Jaeger, E. C. 1944. A Source-book of biological names and terms. C. C. Thomas Publisher, springfield, IL, 256 pp.
Naef, A. 1921/23. Cephalopoda. Fauna e Flora del Golfo di Napoli. Monograph, no. 35. English translation: A. Mercado (1972). Israel Program for Scientific Translations Ltd. IPST Cat. No. 5110/1,2.
Sweeney, M. J. and C. F. E. Roper. Classification, type localities and type repositories of Recent Cephalopoda. Smithson. Contr. Zool., No. 586, Vol.2:561-599.
Young, R. E. and C. F. E. Roper. 1968. The Batoteuthidae, a new family of squid (Cephalopoda; Oegopsida) from antarctic waters. Antarctic Res. Ser. 2: 185-202.
Young, R. E. 1991. Chiroteuthid and related paralarvae from Hawaiian waters. Bull. Mar. Sci. 49: 162-185.
Young, R. E. and R. F. Harman. 1996. Phylogeny of the "enoploteuthid" families. Smithson. Contr. Zool., No. 586, Vol..
Young, R. E. and M. Vecchione. 1996. Analysis of morphology to determine primary sister taxon relationships within coleoid cephalopods. Amer. Malac. Bull. 12: 91-112.
Young, R. E., M. Vecchione and D. Donovan. 1998. The evolution of coleoid cephalopods and their present biodiversity and ecology. South African Jour. Mar. Sci. 20: 393-420.
Title Illustrations

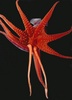
| Scientific Name | Histioteuthis |
|---|---|
| Location | Hawaii |
| View | Oral view showing eight arms and two tentacles |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1995

|
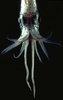
| Scientific Name | Pyroteuthis addolux |
|---|---|
| Location | Hawaii |
| View | Dorsal view showing eight arms and two tentacles |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1995

|
About This Page

University of Hawaii, Honolulu, HI, USA

National Museum of Natural History, Washington, D. C. , USA
Katharina M. Mangold (1922-2003)

Laboratoire Arago, Banyuls-Sur-Mer, France
Page copyright © 2019 , , and Katharina M. Mangold (1922-2003)
 Page: Tree of Life
Decapodiformes . Squids, cuttlefishes and their relatives.
Authored by
Richard E. Young, Michael Vecchione, and Katharina M. Mangold (1922-2003).
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Decapodiformes . Squids, cuttlefishes and their relatives.
Authored by
Richard E. Young, Michael Vecchione, and Katharina M. Mangold (1922-2003).
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 26 March 2019
Citing this page:
Young, Richard E., Michael Vecchione, and Katharina M. Mangold (1922-2003). 2019. Decapodiformes . Squids, cuttlefishes and their relatives. Version 26 March 2019. http://tolweb.org/Decapodiformes/19404/2019.03.26 in The Tree of Life Web Project, http://tolweb.org/










 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site