Dipsacales
Charles D. Bell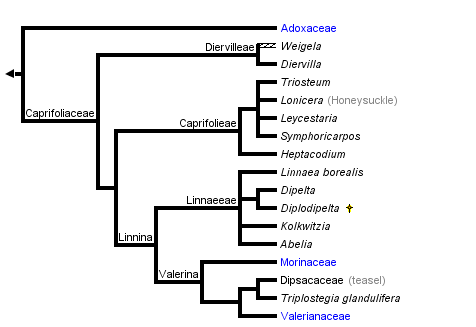


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Dipsacales, with over 1000 species, form a branch within the Asteridae related to the Asterales, Apiales, and several smaller lineages (Bremer et al. 2002).
Knowledge of phylogenetic relationships within Dipsacales has improved dramatically over the last two decades, but especially so within the last few years. Analyses have been based on morphological characters, as well as various molecular datasets, analyzed both separately and in various combinations (Donoghue 1983a; Donoghue et al. 1992; Judd et al. 1994; Backlund and Donoghue 1996; Backlund and Bremer 1997; Kim et al. 1999; Pyck et al. 1999; Pyck and Smets 2000; Pyck 2001; Donoghue et al. 2001; Bell et al. 2001; Pyck et al. 2002; Zhang et al. 2002; Bell and Donoghue 2003).
Overall, these studies show remarkable congruence with respect to the major lineages identified and their relationships to one another. Recently, phylogenetic names have been applied to these major clades (Donoghue et al. 2001; Fig. 1A). The basal split separates Adoxaceae (including Viburnum, Sambucus, and Adoxina, which contains Adoxa and its relatives) from Caprifoliaceae (including Diervilleae, Caprifolieae, Linnaeeae, Morinaceae, Valerianaceae, and Dipsacaceae). Within Adoxaceae, Viburnum is sister to Adoxoideae, which includes Sambucus and the Adoxina clade. Within Caprifoliaceae, Donoghue et al. (2001) provided phylogenetic definitions for the names of two major clades (de Queiroz and Gauthier 1994; Cantino and de Queiroz 2000). The Linnina clade includes Linnaeeae and the Valerina clade, which contains the herbaceous groups Morinaceae, Valerianaceae, and Dipsacaceae. Triplostegia now appears to be more closely related to Dipsacaceae than it is to Valerianaceae (in agreement with Thorne 1983; Peng et al. 1995, but contra Cronquist 1981; Backlund and Donoghue 1996; Backlund and Nilsson 1997; Pyck 2001).
Fossil Record & Molecular Dating
Although the fossil record is not rich for Dipsacales, a number of fossils have been well documented (Muller, 1981; Manchester and Donoghue, 1995; Backlund, 1996). In the Adoxaceae, Sambucus has been reported based on endocarps from the late Eocene to Pliocene of Europe (Reid and Chandler, 1926). Fruits of Dipelta, which are surrounded by distinctive papery bracts, have been described from the late Eocene/early Oligocene (Reid and Chandler, 1926), and those of Heptacodium have been identified from the late Miocene (11.2-5.3 mya) of Japan (Ozaki, 1980). Diplodipelta (Manchester and Donoghue, 1995), from the late Eocene Florissant flora of Colorado (36-35 mya) and several Oligocene sites in western North America, appears to be the sister group of modern Dipelta; both are characterized by enlarged supernumerary bracts that form a wind-dispersed unit. The distinctive winged seeds of Weigela have been reported from the Miocene and Pliocene of Poland (Lancucka-Srodoniowa, 1967), the Oligocene and Miocene of Siberia (Dorofeev, 1963), as well as from the Miocene of eastern Asia (Nikitin, 1976). In Valerianaceae, the unusual fruits ("wing" on the ovary) of Patrinia have been documented from the Miocene to Pliocene of Poland and Russia (Lancucka-Srodoniowa, 1967), as well as from the late Miocene of Japan (Ozaki, 1980). Likewise, Valeriana is known on the basis of fossil fruits from the late Miocene and Pliocene in Europe. Fossils of other Dipsacales have been reported, but are now known to be incorrectly assigned or are considered too unreliable. These include Abelia (Manchester and Habley, 1997) and many specimens attributed to Viburnum and Lonicera.


Diplodipelta from the late Eocene Florissant flora of Colorado. Photograph copyright © Steven Manchester.
Several attempts have been made to estimate the age of Dipsacales. Backlund's (1996) study used a linear regression method with rbcL sequence data and several fossil calibration points, and estimated that Dipsacales originated around 70-60 million years ago (mya), during the late Cretaceous or Early Tertiary. This study assumed a molecular clock for the rbcL data and used the midpoint of major geological time periods for fossil calibrations. In an analysis of 560 species based on a three-gene phylogeny (Soltis et al., 1999; 2000), Wikstrom et al. (2001) used nonparametric rate smoothing (NPRS) to estimate divergence times across angiosperms. They estimated an age for Dipsacales of 81-78 my. Since the purpose of the Wikstrom et al. analysis was to estimate the age of angiosperms as a whole, and of major lineages within angiosperms, the sampling of Dipsacales was poor. The accuracy of the age estimates from both of these analyses may also suffer from the fact that they used the "wrong" topology for Dipsacales based on our current knowledge.
Bell and Donoghue (2005) used recently proposed methods that relax the assumption of rate constancy among lineages (local clocks, nonparametric rate smoothing, penalized likelihood, and Bayesian relaxed clock) to estimate the ages of Dipsacales. Age estimates for Dipsacales varied widely among markers and codon positions, and depended on the fossils used for calibration and method of analysis. Some methods yielded dates for the Dipsacales diversification that appear to be too old (prior to the presumed 125 my [million years] age of eudicots), and others suggested ages that are too young based on well-documented Dipsacales fossils. Concordant penalized likelihood and Bayesian studies imply that Dipsacales originated in the Cretaceous, as did its two major lineages, Adoxaceae and Caprifoliaceae. However, diversification of crown Adoxaceae and Caprifoliaceae mainly occurred in the Tertiary, with the origin of major lineages within these clades mainly occurring during the Eocene. Another round of diversification appears to have occurred in the Miocene. Several radiations, such as Valerianaceae in South America and Dipsacaceae around the Mediterranean, are even more recent. This study demonstrates the wide range of divergence times that can be obtained using different methods and data sets, and cautions against reliance on age estimates based on only a single gene or methodology. Despite this variance, significant conclusions can be made about the timing of Dipsacales evolution (see, Bell and Donoghue, 2005 for more discussion).
Discussion of Phylogenetic Relationships
All phylogenetic analyses have shown that Caprifoliaceae in the traditional sense (consisting of Viburnum, Sambucus, Diervilleae, Caprifolieae, and Linnaeeae) do not form a clade. In the phylogenetic classification of Donoghue et al. (2001) the most significant change is that the name Caprifoliaceae is applied to the clade including Diervilleae, Caprifolieae, and Linnaeeae, as well as the Morinaceae, Valerianaceae, and Dipsacaceae. In the interest of nomenclatural stability these six names have been retained, since in their traditional sense they refer to clades. The classification suggested by Backlund and Pyck (1998; also Backlund and Bremer 1996; APG 1998; Stevens 2002) is rejected because it requires a variety of name changes only for the sake of adjusting ranks (Diervilleae, Caprifolieae, and Linnaeeae are renamed Diervilleaceae, Caprifoliaceae, and Linnaeaceae, respectively), and because it fails to provide names for several well supported clades (e.g., the Linnina clade, which includes Linnaeeae, Morinaceae, Valerianaceae, and Dipsacaceae). Similar criticisms apply to other recently proposed classifications (Benko-Iseppon and Morawetz 2000).
Progress has also been made in elucidating phylogenetic relationships within particular Dipsacales clades. Within Adoxaceae, analyses of several datasets have been reported for Viburnum, including morphological characters (Donoghue 1983b), cpDNA restriction sites (Donoghue and Sytsma 1993), and sequences of the nuclear ribosomal internal transcribed spacer (ITS) region (Donoghue and Baldwin 1993). More recently, analyses have been conducted using the chloroplast locus matK and the nuclear granule-bound starch synthase (waxy) genes (Winkworth and Donoghue 2002, unpublished data). Sambucus has been analyzed using ITS sequences (Eriksson and Donoghue 1998), and Adoxina using morphology and sequences of rbcL and ITS (Liu et al. 2000; Donoghue et al. 2001). Within Caprifoliaceae, Kim and Kim (1999) analyzed Diervilleae (Diervilla and Weigela) using ITS sequences. Triosteum has been studied using ITS and waxy sequences (Gould & Donoghue 2000), and more recently using cpDNA trnL and matK sequences (Cullis-Suzuki, Bell, Winkworth, and Donoghue, unpublished data), and Lonicera is currently under study using ITS and matK (Li & Donoghue 2002, unpublished data). Bell and Donoghue (2002, 2003) have carried out analyses within Morinaceae and Valerianaceae based on trnL, matK, and ITS. For Dipsacaceae, Caputo and Cozzolino (1994) analyzed morphological characters, Mayer and Ehrendorfer (1999, 2000) presented phylogenetic hypothesis for Scabiosa and Pterocephalus and their relatives, and studies are underway using trnL, matK, ITS (Bell and Donoghue, unpublished data) In all, nearly 300 species have now been included in one or another phylogenetic analysis.
To date the single most convincing phylogenetic study of the Dipsacales, both in terms of character number (7593 nucleotide sites) and confident resolution, is that of Bell et al. (2001). However, this study was based entirely on chloroplast DNA sequences, which leaves open the possibility of incongruence with other data sources. Although it is comforting that cpDNA and morphological analyses have yielded very similar results, the addition of data from nuclear genes would be highly desirable.
References
Albach, D. C., P. S. Soltis, D. E. Soltis, and R. G. Olmstead. 2001. Phylogenetic analysis of asterids based on sequences of four genes. Annals of the Missouri Botanical Garden 88:163-212.
Backlund, A. A. & Bremer, K. 1997. Phylogeny of the Asteridae s. str. based on rbcL sequences, with particular reference to the Dipsacales. Plant Systematics and Evolution 207:225-254.
Backlund, A. A. & Bremer, K. 1998. To be or not to be- principles of classification and monotypic plant families. Taxon 47:391-400.
Backlund, A. A. & Donoghue, M. J. 1996. Morphology and phylogeny of the order Dipsacales. In Phylogeny of the Dipsacales, A. A. Backlund, Doctoral Dissertation. Uppsala: Department of Systematic Botany, Uppsala Univ.
Backlund A. A. & Pyck, N. 1998. Diervillaceae and Linnaeaceae, two new families of caprifolioids. Taxon 47:657-661.
Bell, C. D. and M. J. Donoghue. 2003. Phylogeny and biogeography of Morinaceae (Dipsacales) based on nuclear and chloroplast DNA sequences. Organisms, Diversity, and Evolution.
Bell, C. D., Edwards, E. J., Kim, S.-T. & Donoghue, M. J. (2001): Dipsacales phylogeny based on chloroplast DNA sequences. Harvard Papers in Botany 6:481-499.
Blackmore, S. & Cannon, M. J. (1983): Palynology and systematics of Morinaceae. Rev. Palaeobot. and Palyn.40:207-226.
Bremer, K., A. Backlund, B. Sennblad, U. Swenson, K. Andreasen, M. Hjertson, J. Lundberg, M. Backlund, and B. Bremer. 2001. A phylogenetic analysis of 100+ genera and 50+ families of euasterids based on morphological and molecular data with notes on possible higher level morphological synapomorphies. Plant Systematics and Evolution 229:137-169.
Bremer, B., K. Bremer, N. Heidari, P. Erixon, R. G. Olmstead, A. A. Anderberg, M. K?llersj?, and E. Barkhordarian. 2002. Phylogenetics of asterids based on 3 coding and 3 non-coding chloroplast DNA markers and the utility of non-coding DNA at higher taxonomic levels. Molecular Phylogenetics and Evolution 24:274-301.
Cannon, M. J. & Cannon, J. F. M. (1984): A revision of the Morinaceae (Magnoliophyta-Dipsacales). Bull. Brit. Mus. (Nat. Hist.) Bot. 12:1-35.
Caputo, P. & Cozzolino, S. (1994): A cladistic analysis of Dipsacaceae (Dipsacales). Pl. Syst. Evol. 189:41-61.
Cronquist, A. (1988): The Evolution and Classification of Flowering Plants. New York Botanical Garden, Bronx, New York.
Donoghue, M. J., T. Eriksson, P. A. Reeves, and R. G. Olmstead. 2001. Phylogeny and phylogenetic taxonomy of Dipsacales, with special reference to Sinadoxa and Tetradoxa (Adoxaceae). Harvard Papers in Botany 6:459-479.
Donoghue, M. J., R. G. Olmstead, J. F. Smith, and J. D. Palmer. 1992. Phylogenetic relationships of Dipsacales based on rbcL sequences. Annals of the Missouri Botanical Garden 79:333-345.
Donoghue, M. J., Bell, C. D., & Winkworth, R. C. (in press): The evolution of reproductive characters in Dipsacales. Int. J. Plant Sci.
Hofman, U. & Gottmann, J. (1990): Morina L. und Triplostegia Wall. Ex DC. Im vergleich mit Valerianaceae und Dipsacaceae. Bot. Jahrb. Syst. 111:499-553.
Judd, W. S., Sanders, R. W. & Donoghue, M. J. (1994): Angiosperm family pairs preliminary phylogenetiic analyses. Harvard Pap. in Bot. 5:1-51.
Manchester, S. R. & Donoghue, M. J. (1995): Winged fruits of Linnaeeae (Caprifoliaceae) in the Tertiary of western North America: Diplodipelta gen. nov. International Journal of Plant Sciences 156:709-722.
Olmstead, R. G., K.-J. Kim, R. K. Jansen, and S. J. Wagstaff. 2000. The phylogeny of the Asteridae sensu lato based on chloroplast ndhF gene sequences. Molecular Phylogenetics and Evolution 16:96-112.
Peng, C.-I., Tobe, H. & Takahashi, M. (1995): Reproductive morphology and relationships of Triplostegia (Dipsacales). Bot. Jahrb. Syst. 116:505-516.
Pyck, N., Roels, P. & Smets, E. (1999): Tribal relationships in Caprifoliaceae: evidence from a cladistic analysis using ndhF sequences. Syst. Geogr. Pl. 69:145-159.
Pyck, N. & Smets, E. (2000): A search for the position of the seven-son flower (Heptacodium, Dipsacales): combining molecular and morphological evidence. Pl. Syst. Evol. 225:185-199.
Pyck, N., Van Lysebetten, A., Stessens, J. & Smets, E. (2002): The phylogeny of Patrinieae sensu Grabner (Valerianaceae) revisited: additional evidence from ndhF sequence data. Pl. Syst. Evol. 233:29-46.
Roels, P. & Smets, E. (1996): A floral ontogenetic study in Dipsacales. Int. J. Plant Sci. 157:203-218.
Savolainen, V., M. F. Fay, D. C. Albach, A. Backlund, M. van der Bank, K. M. Cameron, S. A. Johnson, M. D. Lled?, J.-C. Pintaud, M. Powell, M. C. Sheahan, D. E. Soltis, P. S. Soltis, P. Weston, W. M. Whitten, K. J. Wurdack, and M. W. Chase. 2000. Phylogeny of the eudicots: a nearly complete familial analysis based on rbcl gene sequences. Kew Bulletin 55:257-309.
Zhang, W-H., Chen, Z-D., Li, J-H., Chen, H-B. & Tang, Y-C. (2003): Phylogeny of the Dipsacales s.l. based on chloroplast trnL-F and ndhF sequences. Mol. Phylo. Evol. 26:176-189.
Title Illustrations

| Scientific Name | Dipsacus fullonum |
|---|---|
| Location | Mt. Shasta (Siskiyou County, California, USA) |
| Comments | Fuller's teasel (Dipsacaceae) |
| Source Collection | CalPhotos |
| Copyright | © 2002 |
| Scientific Name | Lonicera maackii |
|---|---|
| Location | Kedrovaja Pad Natural Reserve, Khasansky distr., Primorsky Territory (Russian Federation) |
| Comments | Amur honeysuckle (Caprifoliaceae) |
| Source Collection | CalPhotos |
| Copyright |
© 1999

|
About This Page
Charles D. Bell

University of New Orleans, New Orleans, Louisiana, USA
Page copyright © 2005 Charles D. Bell
All Rights Reserved.
- Content changed 31 July 2004
Citing this page:
Bell, Charles D. 2004. Dipsacales. Version 31 July 2004 (under construction). http://tolweb.org/Dipsacales/20743/2004.07.31 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site