Effects on the host were studied in Lobatostoma manteri infecting snails. Both naturally and experimentally infected snails were examined. Naturally infected Cerithium moniliferum harbour usually a single parasite coiled up in a cavity formed by the main and (or some?) side ducts of the digestive gland with metaplasia of the duct epithelium, hyperplasia of the interfollicular connective tissue and ameobocytes, and necrosis of some glandular follicles (Fig.1-3) (Rohde, 1972, 1973, 1975).

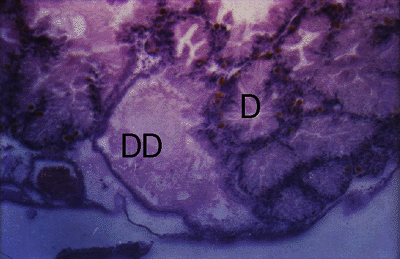
Figure 1. Cerithium moniliferum. Section through digestive gland of uninfected snail showing glandular duct (DD) and glandular follicles (D).

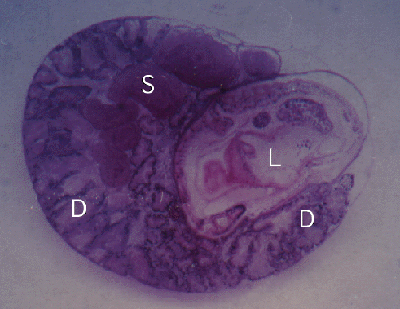
Figure 2. Cerithium moniliferum. Section through snail with a single Lobatostoma manteri (L) in the digestive gland (D). Also note spermducts (S) of snail filled with sperm.

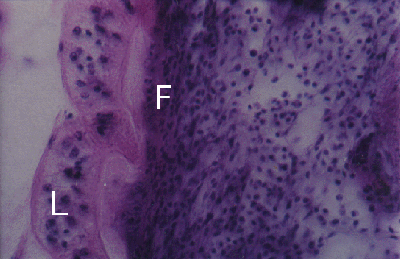
Figure 3. Cerithium moniliferum. Section through adhesive disc (L) of Lobatostoma manteri attached to snail tissue. Note dense fibrous tissue (F) close to adhesive disc.
The snail Peristernia australiensis harbours up to six parasites in the stomach and large digestive ducts of the gland (Fig.4), with a thickening of the subepithelial connective tissue layer (Figs. 4 and 5).

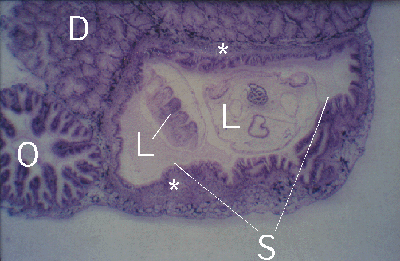
Figure 4. Peristernia australiensis. Section through oesophagus (O), digestive gland (D) and stomach (S) with two Lobatostoma manteri (L). Note thick fibrous layer (*) below stomach epithelium of host.

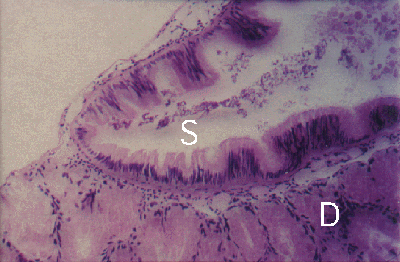
Figure 5. Peristernia australiensis. Section through digestive gland (D) and stomach (S) of uninfected snail. Note: no thick fibrous layer below stomach epithelium of snail.
There was a strong decrease in the relative number of infected snails of both species over a four month period at a heavily infected site at Heron Island, Great Barrier Reef. During the period of high frequency of infection, Cerithium infected with Digenea contained Lobatostoma relatively more frequently than Cerithium without Digenea. Snails with double infections disappeared first. Infection with Lobatostoma did not affect the relative number of egg-producing Cerithium during the period of high frequency of infection.
Planaxis sulcatus and Cerithium moniliferum were experimentally infected with large numbers of eggs. The larvae hatched in the stomach and migrated immediately along the ducts of the digestive gland into the digestive follicles. The larvae fed on the secretion and probably epithelial cells of the follicles. The posterior sucker was used for adhesion to the epithelium and contributed to its erosion. In heavily infected snails, the digestive follicles disappeared gradually and the larvae lived in cavities lined by a flattened epithelium, parts of which show secretory activity. In snails dissected 47-49 and 65-66 days after infection, the cavities were fused, forming several large spaces which communicate with each other; only small parts of the epithelium were still secretory. Concentrations of ameobocytes occurred in the walls of the digestive gland and in the wall between digestive gland and stomach of infected Planaxis. No tissue reactions were seen around the stomach except in the wall between digestive gland and the stomach. In Cerithium with 65-67 days old infection, the cavities contained much detritus and disintegrating cells, the epithelium was practically non-secretory and surrounded by loose connective tissue.



 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site