Myxozoa
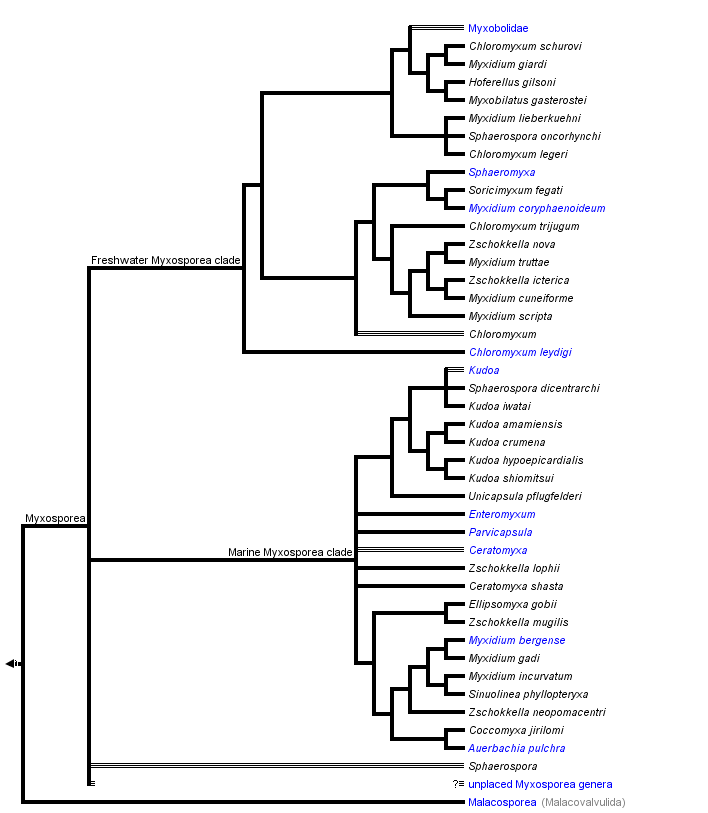


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Members of the Myxozoa are microscopic metazoan parasites with an extremely reduced body. The dimensions of the myxospore, the typical myxozoan stage in fish hosts, range usually between one hundredth and two hundredth of a millimetre. Myxospores consist of several cells, which are transformed to shell valves, nematocyst-like polar capsules with coiled extrudible polar filaments and amoeboid infective germs. Myxospores develop in plasmodia (trophozoites), which can be very large and polysporic (generally histozoic in host tissue) or small and mono- or disporic (coelozoic in organ cavities). Myxozoans are parasites of fish, worms (oligochaetes and polychaetes) and bryozoans. Few representatives were found as parasites of amphibians and reptiles, and recent findings confirmed the ability of myxozoans to infect mammals (Prunescu et al. 2007, Dyková et al. 2007) and birds (Bartholomew et al. 2008). Humans as potential hosts for myxosporea were also reported (Boreham et al. 1998, Moncada et al. 2001), however, myxospores were detected in faecal samples and probably just passed through the digestive tract.
Myxozoa Grassé, 1970 contains two classes: Malacosporea Canning, Curry, Feist, Longshaw et Okamura, 2000 and Myxosporea Bütschli, 1881. Malacosporea includes only two genera (Tetracapsuloides and Buddenbrockia) with a total of three described species. Myxosporea includes about 2200 species in 60 genera.
Wolf and Markiw (1984) discovered myxosporean life cycles altering between two host species – fish and annelid worm. The myxospore is ingested by annelids and then the myxosporean undergoes a schizogony and a gametogony. Finally, the parasite develops into an actinospore, a triradiate myxosporean spore, which infects the vertebrate host. Here, the sporoplasm released from the actinospore divides by endogony, and then presporogenic multiplication of the myxosporean occurs. The life cycle is completed with the development of mature myxospores in sporogonic plasmodia. The annelids are definitive hosts whereas vertebrates are intermediate hosts for Myxosporea.
Myxozoans were considered to be protists for more then one hundred years until the early nineties of 20th century. Then, the phylogenetic analysis of the primal myxosporean SSU rDNA sequence (Smothers et al. 1994) confirmed earlier hypotheses that myxozoans are multicellular organisms (Štolc 1899, Weill 1938) and placed Myxozoa inside Metazoa. However, SSU rDNA data failed to find the correct position of the Myxozoa within metazoan taxa. Myxozoan SSU rDNA appeared to be a fast-evolving sequence resulting in long-branches in phylogenetic trees. Therefore, the SSU rDNA data are insufficient to decide whether Myxozoa are closely related either to Bilateria, Cnidaria (including Polypodium hydriforme) or other taxa (Smothers et al. 1994, Siddall et al. 1995, Hanelt et al. 1996, Siddall and Whiting 1999).
The rediscovery of Buddenbrockia plumatellae, a worm-like animal, as a myxozoan species was an important clue to the origin of Myxozoa (Monteiro et al. 2002). SSU rDNA of this enigmatic worm showed its close relationship to Tetracapsuloides bryosalmonae, and B. plumatellae was assigned to Malacosporea, the sister group to Myxosporea. Consequently Myxozoa were considered to be bilaterians or their close relatives (Monteiro et al. 2002). However, phylogenetic analysis based on sequences of numerous protein-coding genes (Jimenez-Guri et al. 2007) excluded a bilaterian origin of B. plumatellae and suggested Cnidaria as the most closely related taxon to Myxozoa.
The malacosporean Tetracapsuloides bryosalmonae and some myxosporean species are of economic importance causing significant losses of farm-reared fish. T. bryosalmonae causes dangerous proliferative kidney disease of salmonids (Kent et al. 1994). Probably the most serious myxosporean pathogen is Myxobolus cerebralis (whirling disease) which infects cartilage of freshwater salmonids and causes vast losses due to death (Nehring and Walker 1996). The other economically important species are e. g. Enteromyxum leei (enteromyxosis) (Palenzuela et al. 2002), Henneguya ictaluri (proliferative gill diseases) (Pote et al. 2000) and Kudoa thyrsites (post-harvest soft flesh) (Kent et al. 1994).
Discussion of Phylogenetic Relationships
Hypotheses of phylogenetic relationships of Myxozoa are based primarily on the SSU rDNA data. These molecular data helped to confirm the phylogenetic position of Myxozoa inside Metazoa, and they have been a useful tool in discovering the relationships among myxozoan subgroups. The increasing number of myxosporean SSU rDNA sequences and their phylogenetic analyses cast doubt on the traditional taxonomic scheme of Myxosporea. Phylogenetic trees reveal great discrepancies between current taxonomy based on spore morphology (Lom and Dykova 2006) and analyses of molecular data. Particularly, the broader phylogenetic study of Fiala (2006) showed six polyphyletic and two paraphyletic genera and only four myxosporean genera were monophyletic. Moreover, just a fraction of myxosporean species diversity has been sequenced, and there are no sequence data from two thirds of described myxosporean genera. The tree above is based on the SSU rDNA tree after Fiala (2006).
The phylogenetic analyses of myxosporean SSU rDNA revealed unexpected and surprising relationships, e. g. the very close relationship of five species each from different genera (Myxidium giardi, Hofferelus gilsoni, Chloromyxum sp., Zschokkella sp. and Myxobilatus gasterostei); nine Sphaerospora species with known SSU rDNA forming five unrelated lineages; many Myxidium and Zschokkella species branching in different places in the phylogenetic tree, etc. (Holzer et al. 2004, Fiala 2006).
Many polytomies and paraphylies are typical results of analyses of myxosporean phylogeny. It will be a difficult task to find out relevant morphological synapomorphies, which would define a group of phylogenetically closely related myxosporean species. Spores are an abundant source of morphological features, but they are not an appropriate tool for future taxonomic classification, because in the past these characters have been shown not to be congruent with phylogenetic relationships; while vegetative stages cannot offer a sufficient number of morphological characters for taxonomic classification. For instance, one of the current key diagnostic features of Myxosporea, number of polar capsules (PC), is relatively easily changed during evolution as witnessed by:
- the occurrence of Chloromyxum spp. (4 PC) in several different positions on the phylogenetic tree among myxosporeans with 2 PC;
- the placement of Thelohannelus spp. (1 PC) inside the Myxobolus (2 PC) clade with no close relation to Coccomyxa spp. (1 PC) and Auerbachia pulchra (1 PC), which branch near marine Myxidium spp. (2 PC);
- the multiplication of PC in some Kudoa spp. – K. neurophila (5 PC), K. neothunni (6 PC), K. yasunagai (7 PC) and K. permulticapsulata (13 PC!).
This is in contrast to the multivalve-state of myxospores, which appeared only once in the evolution from a marine bivalvulid ancestor (however, the bivalvulid Sphaerospora dicentrarchi clusters inside the multivalvulid Kudoa spp.). In the traditional classification, Multivalvulida and Bivalvulida represent the only two orders of Myxosporea. However, multivalvulid species constitute just a subgroup of the phylogenetically determined “marine clade”, which is together with its sister “freshwater clade” the main lineage in the phylogenetic tree of Myxosporea (Fiala 2006).
References
Anderson, C. L. 1998. Phylogenetic relationships of the Myxozoa. Pages 341–350 in: Evolutionary Relationships among Protozoa. G. H. Coombs, K. Vickerman, M. A. Sleigh and A. Warren, eds. Chapman & Hall, London.
Anderson, C. L., E. U. Canning and B. Okamura. 1998. A triploblast origin for Myxozoa? Nature392:346.
Anderson, C. L., E. U. Canning and B. Okamura. 1999. Molecular data implicate bryozoans as hosts for PKX (Phylum Myxozoa) and identify a clade of bryozoan parasites within the Myxozoa. Parasitology 119(6):555-561.
Bartholomew, J.L., S.D. Atkinson, S.L. Hallett, L.J. Lowenstine, M.M. Garner, C.H. Gardiner, B.A. Rideout, M.K. Keel and J.D. Brown. 2008. Myxozoan parasitism in waterfowl. International Journal for Parasitology 38: 1199-1207.
Boreham, R.E., S. Hendrick, P.J. O'Donoghue, and D.J. Stenzel. 1998. Incidental finding of Myxobolus spores (Protozoa: Myxozoa) in stool samples from patients with gastrointestinal symptoms. Journal of Clinical Microbiology 36: 3728-3730.
Canning, E. U., A. Curry, S. W. Feist, M. Longshaw, and B. Okamura. 2000. A new class and order of myxozoans to accommodate parasites of bryozoans with ultrastructural observations on Tetracapsula bryosalmonae (PKX organism). Journal of Eukaryotic Microbiology 47:456?468.
Canning, E. U. and B. Okamura. 2004. Biodiversity and evolution of the myxozoa. Advances in Parasitology 56:43-131.
Canning E. U., S. Tops, A. Curry, T. S. Wood, and B. Okamura. 2002. Ecology, development and pathogenicity of Buddenbrockia plumatellae Schröder, 1910 (Myxozoa, Malacosporea) (syn. Tetracapsula bryozoides) and establishment of Tetracapsuloides n. gen. for Tetracapsula bryosalmonae. Journal of Eukaryotic Microbiology 49(4):280-295.
Dykova, I., T. Tyml, I. Fiala and J. Lom. 2007. New data on Soricimyxum fegati (Myxozoa) including analysis of its phylogenetic position inferred from the SSU rRNA gene sequence. Folia Parasitologica 54: 272-276.
Fiala I., 2006. The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. International Journal for Parasitology, 36, 1521-1534.
Hanelt, B., D. Van Schyndel, C.M. Adema, L. Lewis and E.S. Loker. 1996. The phylogenetic position of Rhopalura ophiocomae (Orthonectida) based on 18S ribosomal DNA sequence analysis. Molecular Biology and Evolution 13: 1187-1191.
Holzer, A.S., C. Sommerville, R. Wootten. 2004. Molecular relationships and phylogeny in a community of myxosporeans and actinosporeans based on their 18S rDNA sequences. International Journal for Parasitology 34: 1099-1111.
Jiménez-Guri, E., H. Philippe, B. Okamura, P. W. H. Holland. 2007. Buddenbrockia is a cnidarian worm. Science 317(5834):116-118.
Kent, M. L., K. B.Andree, J. L.Bartholomew, M.El-Matbouli, S. S.Desser, R. H.Devlin, S. W.Feist, R. P.Hedrick, R. W.Hoffmann, J.Khattra, S. L.Hallett, R. J. G.Lester, M.Longshaw, O.Palenzeula, M. E.Siddall, and C.Xiao. 2001. Recent advances in our knowledge of the Myxozoa. Journal of Eukaryotic Microbiology 48:395-413.
Kent, M.L., L. Margolis, D.J.Whitaker, G.E. Hoskins and T.E. McDonald. 1994. Review of Myxosporea of importance to salmonid fisheries and aquaculture in British Columbia. Folia Parasitologica 41: 27–37.
Lom J. and I. Dykova. 2006. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitologica 53(1):1-36.
Moncada, L.I., M.C.Lopez, M.I.Murcia, S. Nicholls, F. Leon, O.L. Guio and A. Corredor. 2001. Myxobolus sp., another opportunistic parasite in immunosuppressed patients? Journal of Clinical Microbiology 39: 1938-1940.
Monteiro, A. S., B. Okamura, and P. W. H. Holland. 2002. Orphan worm finds a home: Buddenbrockia is a Myxozoan. Molecular Biology and Evolution 19:968-971.
Nehring, R.B. and P.G. Walker. 1996. Whirling disease in the wild: The new reality in the intermountain West. Fisheries 21: 28-30.
Okamura, B. and E. U. Canning. 2003. Orphan worms and, homeless parasites enhance bilaterian diversity. Trends in Ecology and Evolution 18(12):633-639.
Okamura, B., A. Curry, T. S. Wood and E. U. Canning. 2002. Ultrastructure of Buddenbrockia identifies it as a myxozoan and verifies the bilaterian origin of the Myxozoa. Parasitology 124:215-223.
Palenzuela, O., M.J. Redondo and P. Alvarez-Pellitero. 2002. Description of Enteromyxum scophthalmi gen. nov., sp nov (Myxozoa), an intestinal parasite of turbot (Scophthalmus maximus L.) using morphological and ribosomal RNA sequence data. Parasitology 124: 369-379.
Pote, L.M., L.A. Hanson and R. Shivaji 2000. Small subunit ribosomal RNA sequences link the cause of proliferative gill disease in channel catfish to Henneguya n. sp. (Myxozoa: Myxosporea). Journal of Aquatic Animal Health 12: 230-240.
Prunescu, C.C., P. Prunescu, Z. Pucek and J. Lom. 2007. The first finding of myxosporean development from plasmodia to spores in terrestrial mammals: Soricimyxum fegati gen. et sp n. (Myxozoa) from Sorex araneus (Soricomorpha). Folia Parasitologica 54: 159-164.
Schlegel, M., J. Lom, A. Stechmann, D. Bernhard, D. Leipe, I. Dyková, and M. L. Sogin. 1996. Phylogenetic analysis of complete small subunit ribosomal RNA coding region of Myxidium lieberkuehni: evidence that Myxozoa are Metazoa and related to the Bilateria. Archiv für Protistenkunde 147:1–9.
Siddall, M. E., D. S. Martin, D. Bridge, S. S. Desser, and D. K. Cone. 1995. The demise of a phylum of protists: phylogeny of the Myxozoa and other parasitic cnidaria. Journal of Parasitology 81:961–967.
Siddall, M. E. and M. F. Whiting. 1999. Long-branch abstractions. Cladistics 15:9–24.
Smothers, J. F., C. D. von Dohlen, L. H. Smith, Jr., and R. D. Spall. 1994. Molecular evidence that the myxozoan protists are metazoans. Science 265:1719–1721.
Štolc, A. 1899. Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies. Bulletin International de l'Academie des Sciences de Boheme. 22: 1–12.
Xiao, C. and S. S. Desser. 2000. Cladistic analysis of myxozoan species with known alternating life-cycles. Systematic Parasitology 46:81–91.
Weill, R. 1938. L 'interpretation des Cnidosporidies et Ia valeur taxonomique de Ieur cnidome. Leur cycle comparé ii Ia phase Iarvaire des Narcomeduses Cuninides. Travaux de la Station Zoologique de Wimereaux 13: 727-744.
Whipps, C. M., G. Grossel, R. D. Adlard, H. Yokoyama, M. S. Bryant, B. L. Munday, and M. L. Kent. 2004. Phylogeny of the multivalvulidae (Myxozoa : Myxosporea) based on comparative ribosomal DNA sequence analysis. Journal of Parasitology 90 (3):618-622.
Wolf, K. and M.E. Markiw. 1984. Biology contravenes taxonomy in the Myxozoa: new discoveries show alternation of invertebrate and vertebrate hosts. Science 225: 1449-1452.
Zrzavy, J. 2001. The interrelationships of metazoan parasites: a review of phylum- and higher-level hypotheses from recent morphological and molecular phylogenetic analyses. Folia Parasitologica 48:81-103.
Zrzavy, J. and V. Hypsa. 2003. Myxozoa, Polypodium, and the origin of the Bilateria: The phylogenetic position of "Endocnidozoa" in light of the rediscovery of Buddenbrockia. Cladistics 19(2):164-169.
Information on the Internet
- Myxozoan Network. A network for scientists involved with myxozoan research.
- When is a worm not a worm? When it's a jellyfish. University of Oxford press release about Jiménez-Guri et al. 2007.
Title Illustrations

| Scientific Name | Sinuolinea sp. |
|---|---|
| Location | From urinary bladder of Lophius piscatorius, Norway |
| Acknowledgements | Thanks to Dr. Oleg Ditrich for collecting the urine of Lophius piscatorius |
| Specimen Condition | Live Specimen |
| Identified By | Ivan Fiala |
| Life Cycle Stage | Spore |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 23.5.2008 Ivan Fiala

|
About This Page
Page copyright © 2008
 Page: Tree of Life
Myxozoa.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Myxozoa.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 10 July 2008
Citing this page:
Tree of Life Web Project. 2008. Myxozoa. Version 10 July 2008 (under construction). http://tolweb.org/Myxozoa/2460/2008.07.10 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site