Katablepharids
Katablepharidae
Noriko Okamoto


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxGenus Katablepharis is currently polyphyletic, and species appear both in the freshwater clade and the marine clade that includes three other genera. Katablepharis here is to show the type species K. phoenikoston on the assumption that it's included in the hitherto detected freshwater clade.
Introduction
Katablepharids are heterotrophic flagellates, which have an oval or cylindrically ovate cell with one anterior and one posterior flagellum emerging from a shallow subapical groove.
Katablepharids have long been classified as a class within cryptophytes since the first description by Skuja (1939) until recently, when Vørs et al (1992) emended them as a class incertae sedis. Recently Okamoto and Inouye (2005a, 2005c) demonstrated that they are an independent protistan group distantly related to cryptomonads (algae) and goniomonads (bacterivorous flagellates) based on molecular phylogeny of SSU rDNA and alpha tubulin.
According to the chromalveolata hypothesis, cryptophytes belong to a supergroup Chromalveolata together with heterokontophytes, alveolates, and haptophytes. Horizontal gene transfer of plastid genes favors the single origin of the plastid among the chromoalveolates algae. Since katablepharids are distant sister of cryptophytes, it is interesting to know whether katablepharids still retain some remnant of the secondary plastid.
Characteristics
Katablepharids share some characters with crypromonads and goniomonads, such as the cell shape, two flagella emerging from a subapical groove and the ultrastructurally similar ejectisomes near/inside the goove; however, their morphology, such as a bilayer sheath around cell and flagellar surface, tubular mitochondrial cristae, and the presence of a complex feeding apparatus is quite distinctive.
Discussion of Phylogenetic Relationships
The original description of katablepharids dates back to 1939 by Skuja, when he described Kathablepharis phoenikoston (the type species) from freshwater and K. hyalurus from marine environments. As the early descriptions were based on light microscopical observation, they used to include several protist genera of uncertain affinity, such as Phyllomitus spp. (kinetoplastids) and Cryptaulax spp. (incertae sedis). More than half a century after the first description, Vørs (1999) emended the class to have three genera, Katablepharis, Leucocryptos, and Platychilomonas. Later the new marine genera Hatena (Okamoto & Inouye 2005b, 2005c) and Roombia (Okamoto et al 2009) were established. Hatena is included in a marine katablepharids clade, while Roombia represents the earliest branch of all katablepharids.
Katablepharis officially has 6 freshwater species including the type species K. phoenikoston, and 3 marine species. There are ultrastructural studies on a marine strain “K. clone-G2” that has never been officially identified nor described. Leucocryptos, Platychilomonas, Hatena and Roombia are monospecific genera, all of which are marine environment.
Availability of the culture strains has been limited, and this is why this taxon has long been neglected. Part of the reason are the requirements of the prey cell, which makes the establishment of the culture more complicated. However, because of their phylogenetic and ecological importance, there are continuous efforts to culture these eukarivorous protists.
Environmental Sequences
Since the first molecular data were provided (Okamoto & Inouye 2005a, c), a number of environmental sequences have been assigned to katablepharids (Šlapeta et al 2006). Interestingly, Šlapeta et al (2006) observed a distinct grouping of marine and freshwater species, although there are no clues from the taxon sampling as to the nature of the environmental sequence data. Nevertheless, this still implies the diversity of katablepharids and their ecological importance, as well as a possible need for re-examination of their taxonomy.
Symbiosis in Progress
Hatena arenicola is most likely to be in the midst of plastid acquisition via secondary endosymbiosis (Okamoto and Inouye 2005b, 2005c), alternating “plant” phase and “predator” phase. Most of the cells from the environment are in the “plant” phase, possessing a Nephroselmis sp. as an endosymbiont. In this phase, Nephroselmis sp. looks like a plastid under the light microscope, with the eyespot always located at the apex of the host cell. However, the symbiont still retains its nucleus. Moreover, their cell division cycles have not been harmonized, as the symbiont cell is always inherited by only one of the daughter host cells. After the cell division, the daughter cell which has lost the symbiont (“predator” phase) needs to uptake a new symbiont from the cell apex, where the feeding apparatus resides. After the uptake, the symbiont Nephroselmis sp. needs to re-establish the morphological association of the eyespot with the host cell apex, and needs to be enlarged more than ten-fold in size.
Nomenclatural Problem
There had been an issue about the genus name Katablepharis/Kathablepharis. In the first description, Skuja used the spelling Kathablepharis for the genus name. However, according to the International Code of Botanical Nomencrature (ICBN), this must be corrected as Katablepharis based on the name's etymology (“trailing flagellum” in Greek; kata = downwards, blepharis = eyelash). On the other hand, according to the International Code of Zoological Nomencrature (ICZN), the spelling must be Kathablepharis regardless of the etymology. Recently, Okamoto et al (2009) published a correction of the name to Katablepharis under ICZN.
References
Auer B, Arndt H (2001) Taxonomic composition and biomass of heterotrophic flagellates in relation to lake trophy and season. Freshwater Biol 46(7): 959–972.
Barlow SB, Kugrens P (2002) Cryptomonads from the Salton Sea, California. Hydrobiol 473(1–3): 129–137.
Brandt SM, Sleigh MA (2000) The quantitative occurrence of different taxa of heterotrophic flagellates in Southampton water, UK. Estuar Coast Shelf S 51(1): 91–102.
Clay BL, Kugrens P (1999) Systematics of the enigmatic kathablepharids, including EM characterization of the type species, Kathablepharis phoenikoston, and new observations on K. remigera comb. nov. Protist 150(1): 43–59.
Cleven EJ, Weisse T (2001) Seasonal succession and taxon-specific bacterial grazing rates of heterotrophic nanoflagellates in Lake Constance. Aquat Microb Ecol 23(2): 147–161.
Kim E, Simpson A, Graham LE (2006) Evolutionary relationships of apusomonads inferred from taxon-rich analyses of 6 nuclear encoded genes. Mol Biol Evol 23(12): 2455–2466.
Kugrens P, Lee RE, Corliss JO (1994) Ultrastructure, biogenesis, and function of extrusive organelles in selected nonciliate protists. Protoplasma 181(1–4): 164–190.
Larsen J, Patterson DJ (1990) Some flagellates (Protista) from tropical marine sediments. J Nat Hist 24(4): 801–937.
Lee RE, Kugrens P, Mylnikov AP (1991) Feeding apparatus of the colorless flagellate Katablepharis (Cryptophyceae). J Phycol 27(6): 725–733.
Lee WJ, Patterson DJ (2000) Heterotrophic flagellates (Protista) from marine sediments of Botany Bay, Australia. J Nat Hist 34(4): 483–562.
Okamoto N, Inouye I (2005) The katablepharids are a distant sister group of the Cryptophyta: A proposal for Katablepharidophyta divisio nova/Kathablepharida phylum novum based on SSU rDNA and beta-tubulin phylogeny. Protist 156(2): 163–179.
Okamoto N, Inouye I (2005) A secondary symbiosis in progress? Science 310(5746): 287.
Okamoto N, Inouye I (2006) Hatena arenicola gen. et sp. nov., a katablepharid undergoing probable plastid acquisition. Protist 157(4): 401–419.
Okamoto N, Chantangsi C, Horák A, Leander BS, Keeling PJ (2009) Molecular Phylogeny and Description of the Novel Katablepharid Roombia truncata gen. et sp. nov., and Establishment of the Hacrobia Taxon nov
Shalchian-Tabrizi K, Eikrem W, Klaveness D, Vaulot D, Minge MA, et al. (2006) Telonemia, a new protist phylum with affinity to chromist lineages. P R Soc B 273(1595): 1833–1842.
Shalchian-Tabrizi K, Kauserud H, Massana R, Klaveness D, Jakobsen K (2007) Analysis of environmental 18S ribosomal RNA sequences reveals unknown diversity of the cosmopolitan phylum Telonemia. Protist 158(2): 173–180.
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Anton Leeuw Int J G 81(1–4): 293–308.
Skuja H (1939) Beitrag zur Algenflora Lettlands. II. Acta Horta Botanici Universitatis Latviensis 11/12: 41–169.
Šlapeta J, López-García P, Moreira D (2006) Present status of the molecular ecology of kathablepharids. Protist 157(1): 7–11.
Vřrs N (1992): Ultrastructure and autecology of the marine, heterotrophic flagellate Leucocryptos marina (Braarud) Butcher 1967 (Katablepharidaceae/Kathablepharidae), with a discussion of the genera Leucocryptos and Katablepharis/Kathablepharis. Eur J Protistol 28(4): 369–389.
Yoon HS, Grant J, Tekle YI, Wu M, Chaon BC, Cole JC, Logsdon JM, Patterson DJ, Bhattacharya D, Katz LA (2008) Broadly sampled multigene trees of eukaryotes. BMC Evol Biol 8: 14.
Title Illustrations

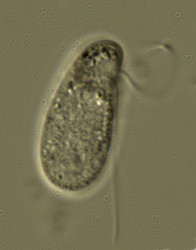
| Scientific Name | Katablepharis |
|---|---|
| Location | observed in freshwater sediments in the vicinity of Broome, Western Australia |
| Source | Kathablepharis |
| Source Collection | Micro*scope |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright | © Angelika Preisfeld and D. J. Patterson |
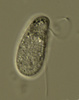
| Scientific Name | Platychilomonas psammobia |
|---|---|
| Location | material collected in marine sediments of Botany Bay (Sydney, Australia) |
| Creator | Won Je Lee |
| Source | Platychilomonas psammobia |
| Source Collection | Micro*scope |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.5. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.5.
|
| Copyright | © Dr. Josef Brief |
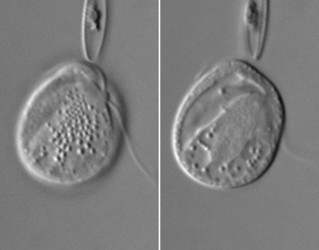
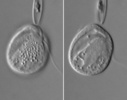
| Scientific Name | Roombia truncata |
|---|---|
| Location | Blomidon Beach, Nova Scotia |
| Specimen Condition | Live Specimen |
| Identified By | Noriko Okamoto |
| Behavior | Crawling on the surface |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0.
|
| Copyright |
© 2009

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.

University of British Columbia, BC, Canada
Correspondence regarding this page should be directed to Noriko Okamoto at
Page copyright © 2009
 Page: Tree of Life
Katablepharids. Katablepharidae.
Authored by
Noriko Okamoto.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Katablepharids. Katablepharidae.
Authored by
Noriko Okamoto.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 28 October 2009
- Content changed 28 October 2009
Citing this page:
Okamoto, Noriko. 2009. Katablepharids. Katablepharidae. Version 28 October 2009 (under construction). http://tolweb.org/Katablepharids/2413/2009.10.28 in The Tree of Life Web Project, http://tolweb.org/







 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site