Hematozoa
Aconoidasida
Jan Šlapeta and Victoria Morin-Adeline

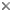
This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Hematozoa Vivier, 1982 (syn. Aconoidasida Mehlhorn, Peters & Haberkorn, 1980)
The phylum Apicomplexa contains a group of blood parasites known as haematozoans. The apical complex of these blood parasites of vertebrates is without the conoid, except in ookinetes of some species of Haemospororida. They are heterxenous in their life cycle–they use vertebrate hosts for asexual reproduction while making use of bloodsucking invertebrate vectors to complete their sexual lifecycle (Lee et al., 2000, Levine, 1973). Hematozoa consist of two principal groups: Piroplasmida and Haemospororida.
Haemospororida use dipteran vectors excusively for transmission (Levine, 1973). By morphological identification alone, over 200 distinct avian haematozoan species of the order Haemospororida Danilewsky 1885, which contain the genera Plasmodium, Leucocytozoon and Haemoproteus, have been described (Bensch et al., 2009). However, it is increasingly evident that the use of light microscopy alone for diagnosis and accurate identification of these blood parasites is subject to many limitations (Criado-Fornelio et al., 2009). With increased use of genetic methods to characterise such organisms, the known diversity of these parasites has increased dramatically (Bensch et al., 2009, Bensch et al., 2000). As a result, the specialist website MalAvi (Avian Malaria Initiative) has been created to document and manage redundancy found in the rapidly increasing genetic data being produced. As an indication of diversity, the database currently lists close to 900 haemosporidian lineages from around 600 host species worldwide (Bensch et al., 2009).
Compared to Haemospororida, a lesser number of species from the order Piroplasmida is known, with those from the genera Babesia and Theileria being the most significant to veterinary and human medicine (Levine, 1973). These parasites are transmitted by tick vectors and infect circulatory cells of vertebrate hosts (Levine, 1973). Tick control is advocated to control the prevalence of these diseases, and some countries have turned to eradication campaigns to eliminate these diseases (Barre et al., 2011, Simuunza et al., 2011)
Blood parasites belonging to this group can be easily considered the most eminent topic of most parasitological research undertaken as they are often used as models for studying patterns of parasite-host interactions within ecosystems, as well as other aspects such as virulence evolution and the pathogenesis inflicted on their vertebrate hosts (Bensch et al., 2000, Hamilton and Zuk, 1982, Moller and Arriero, 2008, Hellgren et al., 2008). Infections in wild bird populations is thought to result merely in a reduction in host fitness (Hamilton and Zuk, 1982). Contrary to this phenomenon, at least one million people die annually from the malaria causing parasite Plasmodium spp. over the 109 countries known to contain both hosts and the parasite (Manguin et al., 2010).
Characteristics
- Parasites of the order Piroplasmorida are generally smaller in size compared to those of the order Haemospororida (Levine, 1973).
- Asexual reproduction stages are seen in invertebrate hosts, while the sexual reproduction stages are seen in vertebrate hosts (Lee et al., 2000)
- Both orders of parasites infect erythrocytes intracellularly and Piroplasmorida additionally infect other circulatory cells (Lee et al., 2000, Levine, 1973)
- Haemospororida has an exoerythrocyte stage not seen in Piroplasmorida (Lee et al., 2000)
- The conoid structure of the apical complex is absent and secretory organelles are reduced in number in Piroplasmorida compared to Haemospororida (Baum et al., 2008, Moltmann et al., 1983a, Levine, 1973)
- Parasites of the order Piroplasmorida and Haemospororida posses motile zygotes known as kinetes and ookinetes respectively (Lee et al., 2000)
- Kinetes and ookinetes develop into sporonts which transformsinto infective sporozoites within the salivary glands of the invertebrate vector (Moltmann et al., 1983b)
- The blood parasite's survival relies heavily on the interaction between its vertebrate host and invertebrate hosts as there are no environmental stages in its life cycle (Moller and Arriero, 2008, Svensson and Ricklefs, 2009).
Hosts
Haemoproteus spp. and Leucocytozoon spp. complete their sexual phases in insects (Diptera) other than mosquitoes; however genetic detection in the gut of mosquitoes has been documented and was dismissed as it may only represent fed material from an infected host (Levine, 1973, Njabo et al., 2011). In vertebrates, both of these parasites infect various avian species, Haemoproteus spp has also been documented in several reptile species (Hellgren et al., 2008, Levine, 1973).
Definitive hosts used by Plasmodium ssp. to produce infective sprozoites belong to four mosquito genera, namely Aedes, Coquillettidia, Culex and Mansonia (Njabo et al., 2011). In vitro investigations have shown that Drosophila melanogaster has potential to cater the asexual stages of Plasmodium, however evidence suggest that zoites are destroyed and it is unlikely that these flies are implicated in transmitting the parasites (Schneider and Shahabuddin, 2000). Like the other two genera of heamatozoans, Plasmodium parasitise a vast array of avian, reptile and mammal species in addition to humans (Levine, 1973).
Parasites of the order Piroplasmida parasitise a large variety of mammals and birds, but less so reptiles and are thought to be the leading drawback to livestock farming in warmer climates that support vector distribution (Lee et al., 2000, Simuunza et al., 2011).
Theilera and Babesia are considered the two main genera of medical importance (Ros-Garcia et al., 2011). These parasites are common in wild populations worldwide, which act as a reservoir for domestic animals and humans alike (Maamun et al., 2011, Barre et al., 2011, Samokhvalov et al., 2010).
Life Cycle
General Life Cycle
Haemosporidians have very complicated heteroxenous life cycles involving a vertebrate and invertebrate host.
Typical Haemospororida lifecycle depicted by the genus Plasmodium
Vertebrate host: The cycle is initiated when a Plasmodium-infected mosquito injects infective sporozoites into the vertebrate’s circulatory system during feeding. The sporozoites enter liver parenchyma cells, localise in a protective parsitophorous vacuole and begin an asexual reproduction phase known as primary exoerythrocytic schizogony which results in merozoites (Lee et al., 2000, Silvie et al., 2008). Entry into the liver is mediated by interaction of sporozoite surface proteins with surface proteogylcans of liver cells (Silvie et al., 2008). Trophozoites feed on the host cell cytoplasm and once mature, a round of schizogony initiates to produce countless numbers of merozoites which leave the hepatocytes through the rupture of liver cells and enter the circulatory system (Prudencio et al., 2006). The newly released parasites then invade erythrocytes to commence another phase of the malaria life cycle known as the erythrocytic cycle (Lee et al., 2000, Silvie et al., 2008). For a second time, the merozoite metamorphoses into a feeding trophozoite and undergoes an erythrocytic schizogony stage which results in 8-16 merozoites which are released to enter new blood cells (Cox, 2010). This cycle is repeated until a trophozoite transforms into micro and macro gametocytes, that is, male and female gametes respectively (Lee et al., 2000).

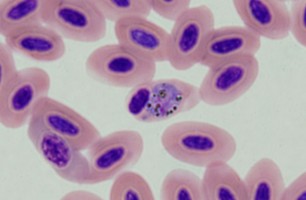
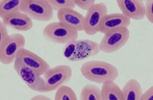
Plasmodium sp. in the brain of Australian magpie blocking a blood capillary in the cerebellum. © Jan Slapeta
Invertebrate host: The sexual phase is initiated when a female anopheline mosquito ingests the gametocytes from the blood of a vertebrate (Cox, 2010). The gametocytes are released from the erythrocytes and undergo further maturation until they are able to fertilise in the lumen of the mosquito intestinal tract to produce a motile zygote called an ookinete (Cox, 2010). The ookinete eventually travels to the hemocoel cavity where it turns into an oocyst in which sporozoites are formed and localise in the mosquito salivary glands, ready to be injected back into the vertebrates blood stream to commence the cycle again (Cox, 2010).
Typical Piroplasmorida life cycle exemplified by Babesia
Invertebrate hosts: Babesia is ingested by a tick when it feeds on an infected vertebrate host and the parasites are eventually phatocytized by the tick epithelial cells where they develop into amoeboid cells directly within the cytoplasm (Lee et al., 2000, Ribeiro et al., 2009). These are then capable of further division and eventually all the progeny elongate to form metozoite-like cells known as vermicules, some of which can enter tick ovaries and localise in developing eggs (Lee et al., 2000). They then migrate to the salivary glands, where asexual multiple fission occurs to produce a large amount of merozoites that are injected into a vertebrate host upon feeding (Lee et al., 2000).
Vertebrate hosts: Upon injection of Babesia merozoites into the vertebrate hosts parasites immediately invade erythrocytes and reside in a parasitophorous vacuole, which they then escape to subsist freely within the erythrocyte cytoplasm (Gohil et al., 2010).
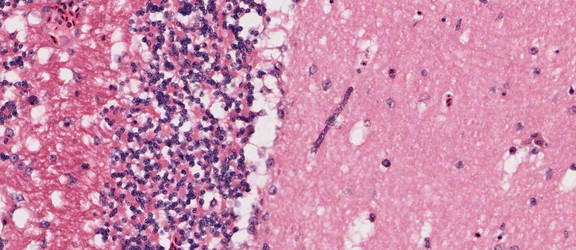
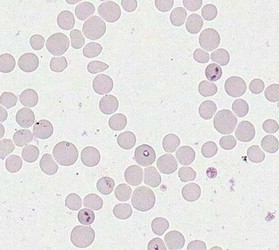 Merozoites of Babesia bovis inside calf's erytrocytes. B. bovis is principle agent causing tick fever in susceptible cattle. © Jan Slapeta | 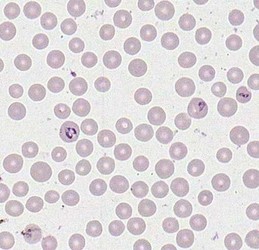 Babesia bigemina merozoites are latger and in acute angle compare to those in B. bovis. This parasites also causes tick fever in susceptible cattle. © Jan Slapeta |
The intraeryothocytic parasite is then able to alter red blood cell properties to allow for longevity of the cells and avoid destruction by the spleen (Gohil et al., 2010). It then feeds off the host cell and transforms into trophozoites which undergo binary fission multiplication to form merozoites (Lee et al., 2000). These merozoites are taken up by invertebrate hosts where the asexual cycle continues. The presence of a sexual stage of the cycle is doubtful for many species (Levine, 1973).
Discussion of Phylogenetic Relationships
According to Lee et al. (2nd ed. 2000) the hematozoan blood parasites are in class Hematozoa (syn. Aconoidasida), this class consists of two orders; Piroplasmida and Haemospororida (Lee et al., 2000). The former only contains one family, which includes parasites of the genera Babesia and Theileria (Lee et al., 2000). Similarly, the latter order is made up of a single family of parasites which contain the most veterinary significant genera of Plasmodium, Haemoproteus and Leucocytozoon (Lee et al., 2000).
Class: Hematozoa Vivier, 1982 (syn. Aconoidasida Mehlhorn, Peters and Haberkorn, 1980)
Characterised by secondarily incomplete apical complex, conoid absent in asexual motile stages (some motile zygotes - ookinetes with conoid); macrogametes and microgametes forming independently; heteroxenous.
Order: Haemospororida Danielewski, 1885
Motile zygote - ookinete with conoid; flagellated microgametes produced by schizogony; oocyst with sporozoites.
Order: Piroplasmorida Wenyon, 1926
Conoid and flagella absent in all stages; piriform, round, rod-shaped or amoeboid; no oocyst; sexual stages still uncertain but probably associated with the formation of the large axopodium-like stages.
Molecular phylogeny revealed monophyly of Hematozoa (syn. Aconoidasida). Similarly piroplasms and haemosporidia are monophyletic sister groups.
Recently, Hematozoa became the home for one of the longest-standing enigmata - Nephromyces (Saffo et. al., 2010). Nephromyces are endosymbiotic marine protists, whose classification was uncertain ever since they were described in the 19th-century. These protists inhabit the lumen of a ductless, urate- and calcium oxalate-rich organ of uncertain function (a.k.a. renal sac), in molgulid ascidian tunicates (Urochordata: Chordata). These protists are ubiquitous nonhereditary symbionts, transmitted horizontally to new hosts. Based on rDNA and some apicomplexa-like ultrastructure, Nephromyces form a monophyletic group with Cardiosporidium cionae, a parasite found in the pericardial bodies of an ascidian, Ciona intestinalis (Ciancio et al., 2008; Saffo et al., 2010). Together these protists are likely part of Hematozoa (Aconoasida) based on their phylogenetic position in SSU rDNA analyses.
References
Barre, N., Happold, J., Delathiere, J. M., Desoutter, D., Salery, M., de Vos, A., Marchal, C., Perrot, R., Grailles, M. & Mortelecque, A. 2011. A campaign to eradicate bovine babesiosis from New Caledonia. Ticks and Tick-borne Diseases, 2, 55-61.
Baum, J., Gilberger, T. W., Frischknecht, F. & Meissner, M. 2008. Host-cell invasion by malaria parasites: insights from Plasmodium and Toxoplasma. Trends in Parasitology, 24, 557-563.
Bensch, S., Hellgren, O. & Perez-Tris, J. 2009. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources, 9, 1353-1358.
Bensch, S., Stjernman, M., Hasselquist, D., Ostman, O., Hansson, B., Westerdahl, H. & Pinheiro, R. T. 2000. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proceedings of the Royal Society of London Series B-Biological Sciences, 267, 1583-1589.
Ciancio, A., Scippa, S., Finetti-Sialer, M., De Candia, A., Avallone, B., De Vincentiis, M. 2008. Redescription of Cardiosporidium cionae (Van Gaver and Stephan, 1907) (Apicomplexa: Piroplasmida), a plasmodial parasite of ascidian haemocytes. Eur J Protistol. 44(3):181-96.
Cox, F. E. 2010. History of the discovery of the malaria parasites and their vectors. 3.
Criado-Fornelio, A., Buling, A., Casado, N., Cimenez, C., Ruas, J., Wendt, L., da Rosa-Faria, N., Pinheiro, M., Rey-Valeiron, C. & Barba-Carretero, J. C. 2009. Molecular characterization of arthropod-borne hematozoans in wild mammals from Brazil, Venezuela and Spain. Acta Parasitologica, 54, 187-193.
Gohil, S., Kats, L. M., Sturm, A. & Cooke, B. M. 2010. Recent insights into alteration of red blood cells by Babesia bovis: moovin' forward. Trends in Parasitology, 26, 591-599.
Hamilton, W. & Zuk, M. 1982. Heritable true fitness and bright birds - A role for parasites. 218, 4570.
Hellgren, O., Bensch, S. & Malmqvist, B. 2008. Bird hosts, blood parasites and their vectors- associations uncovered by molecular analyses of blackfly blood meals. Molecular Ecology, 17, 1605-1613.
Lee, J. J., Leedale, G. F. & Bradbury, P. (eds.) 2000. An illustrated guide to the Protozoa, Kansas: Society of Protozoologists.
Levine, N. D. 1973. The Apicomplexa and the coccidia proper. Protozoan parasites of domestic animals and man. 2nd ed. Minneapolis: Burgress Publishing Company.
Maamun, J. M., Sulemant, M. A., Akinyi, M., Ozwara, H., Kariuki, T. & Carlsson, H. E. 2011. Prevalence of Babesia microtin in free-range baboons and African green monkeys Journal of Parasitology, 97, 63-67.
Manguin, S., Bangs, M. J., Pothikasikorn, J. & Chareonviriyaphap, T. 2010. Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infection Genetics and Evolution, 10, 159-177.
Moller, A. P. & Arriero, E. 2008. Host ecology and life-history traits associated with blood parasite species richness in birds. Journal of Evolutionary Biology, 21, 1504-1513.
Moltmann, U. G., Mehlhorn, H., Schein, E., Rehbein, G., Voigt, W. P. & Zweygarth, E. 1983a. Fine structure of Babesia equi Laveran, 1901 within lymphocutes and erythorocytes of horses: and in vivo and in vitro study. Journal of Parasitology, 69, 111-120.
Moltmann, U. G., Mehlhorn, H., Schein, E., Voigt, W. P. & Friedhoff, K. T. 1983b. Ultrastructural study on the development of Babesia equi (Coccidia: Piroplasmia) in the salivary glands of its vector ticks. Journal of Protozoology, 30, 218-225.
Njabo, K. Y., Cornel, A. J., Bonneaud, C., Toffelmier, E., Sehgal, R. N. M., Valkiunas, G., Russell, A. F. & Smith, T. B. 2011. Nonspecific patterns of vector, host and avian malaria parasite associations in a central African rainforest. Molecular Ecology, 20, 1049-1061.
Prudencio, M., Rodriguez, A. & Mota, M. M. 2006. The silent path to thousands of merozoites: the Plasmodium liver stage. Nature Reviews Microbiology, 4, 849-856.
Ribeiro, M. F. B., Bastos, C. V., Basconcelos, M. M. C. & Passos, L. M. F. 2009. Babesia bigemina:in vitro multipication of sporokinetes in Ixodes scapularis (IDE8) cells. Experimental Parasitology, 122, 192-195.
Ros-Garcia, A., M'Ghirbi, Y. M., Bouattour, A. & Hurtado, A. 2011. First detection of Bacesia occultans in Hyalomma ticks from Tunisia. Parasitology, 138, 578-582.
Samokhvalov, M. V., Kovalevskii, Y. V., Korenberg, E. I., Morozov, A. V., Kuzikov, I. V. & Sheftel, B. I. 2010. Small mammals as potential reservoir hosts of Babesia microti in the Middle Urals. Biology Bulletin, 37, 748-752.
Saffo, M.B., McCoy, A.M., Rieken, C., Slamovits, C.H. 2010. Nephromyces, a beneficial apicomplexan symbiont in marine animals. Proc Natl Acad Sci U S A. 107(37):16190-5.
Schneider, D. & Shahabuddin, M. 2000. Malaria parasite development in a Drosophila model. Science, 288, 2376-2379.
Silvie, O., Mota, M. M., Matuschewski, K. & Prudencio, M. 2008. Interactions of the malaria parasite and its mammalian host. Current Opinion in Microbiology, 11, 352-359.
Simuunza, M., Weir, W., Courcier, E., Tait, A. & Shiels, B. 2011. Epidemiological analysis of tick-borne diseases in Zambia. Veterinary Parasitology, 175, 331-342.
Svensson, L. M. E. & Ricklefs, R. E. 2009. Low diversity and high intra-island variation in prevalence of avian Haemoproteus parasites on Barbados, Lesser Antilles. Parasitology, 136, 1121-1131.
Title Illustrations

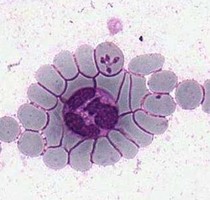
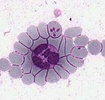
| Scientific Name | Theileria ornithorhynchi |
|---|---|
| Comments | blood of a platypus with Theileria ornithorhynchi |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Jan Šlapeta

|
| Scientific Name | Plasmodium sp. |
|---|---|
| Location | Belrose, NSW, Australia |
| Comments | Plasmodium sp. in blood smear from an Australian Magpie (Gymnorhina tibicen) from Belrose, NSW (SHF0009, bird infected with Haemoproteus / Plasmodium) |
| Specimen Condition | Dead Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Jan Šlapeta

|
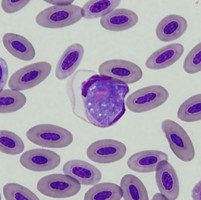
| Scientific Name | Leucocytozoon sp. |
|---|---|
| Location | Turramurra, NSW, Australia |
| Comments | Blood smear from a Pied Currawong (Strepera graculina) from Turramurra, NSW (SHF0006, Leucocytozoon sp.) |
| Specimen Condition | Dead Specimen |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© Jan Šlapeta

|
About This Page
This page is being developed as part of the Tree of Life Web Project Protist Diversity Workshop, co-sponsored by the Canadian Institute for Advanced Research (CIFAR) program in Integrated Microbial Biodiversity and the Tula Foundation.
Jan Šlapeta

Faculty of Veterinary Science, University of Sydney, New South Wales, Australia
Victoria Morin-Adeline

Faculty of Veterinary Science, University of Sydney, Australia
Correspondence regarding this page should be directed to Jan Šlapeta at
Page copyright © 2011 Jan Šlapeta, Victoria Morin-Adeline, and Victoria Morin-Adeline
 Page: Tree of Life
Hematozoa . Aconoidasida .
Authored by
Jan Šlapeta and Victoria Morin-Adeline.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Hematozoa . Aconoidasida .
Authored by
Jan Šlapeta and Victoria Morin-Adeline.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 16 September 2008
- Content changed 18 May 2011
Citing this page:
Šlapeta, Jan and Victoria Morin-Adeline. 2011. Hematozoa . Aconoidasida . Version 18 May 2011 (under construction). http://tolweb.org/Hematozoa/68058/2011.05.18 in The Tree of Life Web Project, http://tolweb.org/





 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site