Heliconiini
Passion-vine Butterflies
Margarita Beltrán, Chris Jiggins, Niklas Wahlberg, and Andrew V. Z. Brower

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Heliconiini or passion-vine butterflies, have played a key role in understanding evolutionary biology (e. g. mimicry) and ecology (e. g. mutualism between insects and plants), and it would be difficult to point a group of neotropical butterflies that have contributed more to our knowledge of the biological processes in the tropics (See Brown 1981 for review). The derived members of the tribe have undergone rapid speciation and divergence, while also exhibiting impressive mimetic convergence in wing patterns.

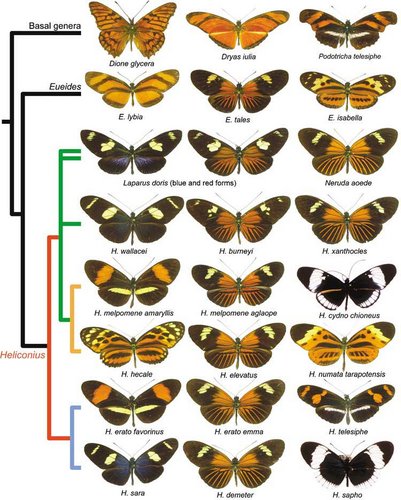
A sample of the morphological diversity of wing patterns in Heliconius and related genera. Each row represents a phylogenetic clade in the tribe Heliconiini. © Chris Jiggins and Mathieu Joron
The figure highlights the rampant pattern diversification within clades and species, and mimicry between clades. Reconstruction of ancestral wing patterns is difficult for such rapidly evolving traits. It may be the case that the orange-rayed pattern is ancestral, in which case the other mimetic patterns are convergent derived patterns. However, it has also been suggested that ancestral Heliconius were more similar to the so-called ‘postman’ pattern of H. melpomene amaryllis, in which case the rayed pattern has evolved repeatedly (Sheppard et al. 1985; Joron et al. 2006).
Etymology: Linnaeus created Heliconius as a subsection of Papilio, to contain delicate butterflies not suited, as were Danaidas and Swallowtails, to being named after the combatants from the Iliad (Turner 1967, 1976). They were mainly named after muses (melpomene, erato, egeria), graces (charithonia) and female deities (doris). In continuation with the tradition, later authors have continued the association with poetry, arts and deities using names like Neruda (a Chilean Poet) and pavana (a Hindu god) (see each species for more information).
Characteristics
Early stages: The larvae of all heliconiines are spiny, have two spines on the head capsule, and can be either solitary or gregarious. Many species have irritant spines that probably discourage predators. The placement of eggs by ovipositing females is correlated with the systematic subdivisions within the Heliconiinae: some lay eggs on tendrils, stipules, leaf tips, or leaves, whereas others lay egg masses generally on old leaves of the host plants (see each species for more details). Such oviposition behaviour is thought to have influenced the leaf shape in Passiflora (Gilbert, 1975) and led to the evolution of egg mimics produced on the stipules and/or leaves of some Passiflora species. These plant-evolved egg mimics inhibit ovipositing females from using the plant to lay eggs.
The pupae of heliconiines are spiny in so-called advanced genera and non-spiny in the basal groups (see adult stage for division of groups). They are suspended with the body held either horizontal or vertical to the pupation substrate (see each species for more details) (Brown, 1981; DeVries, 1997).
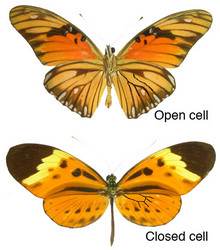
Adult: Agraulis, Dione, Podotricha, Dryadula, and Dryas, termed the “basal” genera by Brown (1981), have the wing venation of the discal cell of the hind wing open (this diagnostic feature is a symplesiomorphy, and the "group" is paraphyletic). These ‘open cell’ heliconiines are relatively palatable, and so generally fast flying to avoid predation. Also, their highly dispersive populations are associated with open sunny habitats, where they visit unspecialised butterfly-pollinated flowers with short corollas and large floral displays (e.g. Lantana).
The remaining genera Eueides, Neruda, and Heliconius (we here subsume the genus Laparus into Heliconius), termed the ‘advanced genera’ by Brown (1981), are the most diverse in terms of numbers of species. All of these possess a closed hind wing discal cell. Their wing patterns differ from the general nymphaline ground plan by great simplification and loss of many elements, as well as by the appearance of several novel mimetic patterns. The ‘closed-cell’ genera, Eueides, Neruda and Heliconius are relatively unpalatable, aposematic, and slow flying. Heliconius also feed on pollen from specialised butterfly-pollinated flowers such as Psiguria.
Discussion of Phylogenetic Relationships
In the last sixty years seven major studies have addressed the systematics of the passion-vine butterflies or Heliconiini (Michener, 1942; Emsley, 1963, 1965; Brown, 1981; Brower, 1994a; Brower & Egan, 1997; Penz, 1999). These phylogenetic hypotheses are in conflict with one another, in particular with regard to the relationships among the genera Heliconius, Eueides, Neruda and Laparus, leading to disagreement over the origin of the derived behavioural trait, pollen feeding. The most recent attempt to resolve the phylogenetic relationships in the group, using molecular markers, showed that Heliconius is paraphyletic, with Laparus doris and Neruda falling within the genus (Beltran et al., 2007). This analysis was based on molecular sequence data for 3 mtDNA and 4 nuclear gene regions. The results would imply a single origin for pollen feeding but with a loss of the trait in Neruda. However different genes are not congruent in their placement of Neruda, such that statistical analysis implies that the traditional placement of Neruda as a sister group to Heliconius cannot be ruled out. Hence, we here place Neruda as sister to Heliconius, but subsume the monobasic genus Laparus into Heliconius (Heliconius doris).
The most recent morphological analysis of the group (Penz, 1999) differs from the tree presented here in three aspects: (1) the relationships among Podotricha, Dryadula, Dryas and Philaethria; (2) the relationships among Laparus, Neruda, Eueides and Heliconius; and (3) the resolution of basal nodes of the tribal tree. We would therefore caution that the relationships presented here might change given more data – either morphological analysis with more complete species sampling, or molecular data with more fast-evolving nuclear gene regions.
Note that we here consider the ‘passion vine butterflies’ as a tribe, Heliconiini, although others have previously ranked them as a sub-tribe Heliconiina (or "Heliconiiti") (Harvey, 1991; Brower and Egan, 1997; Brower, 2000; Beltrán et al. 2007).
Habits
The brightly coloured adults are conspicuous components of virtually all Neotropical habitats below the paramo vegetation type, and are found in all microhabitats in neotropical forests, although most are clearly more abundant in second growth and disturbed forest. All species feed on flower nectar, and the genus Heliconius is the only group of butterflies known to have developed a highly specialized pollen-feeding behaviour (see Heliconius page for more details).
Many heliconiines roost gregariously. The roosts are composed of many individuals of the same species, occasionally with other mimetic species. Individuals return every night to the same spot to sleep (Brown, 1981, DeVries, 1997) (see each species for more details).
Hostplant: The hostplants for the subfamily are almost exclusively in the Passifloraceae, and to a limited extent in the Turneraceae. The restricted hostplant relationships have given rise to the name “passion-vine butterflies”. The close association of heliconiines and Passifloraceae has been important in the understanding of coevolution between insects and plants (Benson et al., 1976) and in assessing the role of plant defences against herbivores (Gilbert, 1971)(see each species for more details).
References
Beltrán M, Jiggins CD, Brower AVZ, Bermingham E, Mallet M. 2007. Do pollen feeding, pupal-mating and larval gregariousness have a single origin in Heliconius butterflies? Inferences from multilocus DNA sequence data. Biological Journal of the Linnean Society 92:221-239.
Benson WW, Brown KS, Gilbert LE 1976. Coevolution of plants and herbivores: passion flower butterflies. Evolution 29, 659-680.
Brower AVZ. 1994. Phylogeny of Heliconius butterflies inferred from mitochondrial DNA sequences (Lepidoptera: Nymphalidae). Molecular Phylogenetics and Evolution 3: 159-174.
Brower, AVZ. 2000. Phylogenetic relationships among the Nymphalidae (Lepidoptera) inferred from partial sequences of the wingless gene. Proc. R. Soc. Lond. B 267, 1201-1211.
Brower AVZ, and Egan MG. 1997. Cladistics of Heliconius butterflies and relatives (Nymphalidae: Heliconiini): the phylogenetic position of Eueides based on sequences from mtDNA and a nuclear gene. Proc. R. Soc. Lond. B 264: 969-977.
Brown KS, Jr. 1981. The biology of Heliconius and related genera. Ann. Rev. Entomol. 26: 427-456.
DeVries P. J. 1997 The Butterflies of Costa Rica and Their Natural History, Volume I: Papilionidae, Pieridae, Nymphalidae Princeton University Press, Baskerville, USA.
Emsley M. 1963. A morphological study of imagine Heliconiinae (Lep.: Nymphalidae) with a consideration of the evolutionary relationships within the group. Zoologica NY 48: 85-130.
Emsley MG. 1965. Speciation in Heliconius (Lep., Nymphalidae): morphology and geographic distribution. Zoologica, New York 50: 191-254.
Ehrlich PR, Gilbert LE 1973. Population structure and dynamics of the tropical butterfly Heliconius ethilla. Biotropica 5, 69-82.
Gilbert LE. 1971. Butterfly-plant coevolution: has Passiflora adenopoda won the selectional race with heliconiine butterflies? Science 172, 585-586.
Harvey, DJ. 1991. Higher classification of the Nymphalidae. In: Nijhout, HF. (Ed.) The development and evolution of butterfly wing patterns. Smithsonian Institution Press, Washington D. C., pp. 255-273.
Joron, M. Jiggins, CD., Papanicolaou, A., McMillan, WO. 2006. Heliconius wing patterns: an evo-devo model for understanding phenotypic diversity. Heredity 97:157-167.
Lamas G, Callaghan C, Casagrande MM, et al. 2004 Hesperioidea and Papilionoidea Association for Tropical Lepidoptera, Gainesville, Florida.
Mallet J, Gilbert LE. 1995. Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biological Journal of the Linnean Society 55, 159-180.
Michener CD. 1942. A generic revision of the Heliconiinae (Lepidoptera, Nymphalidae). Amer. Mus. Novit. 1197: 1-8.
Penz CM. 1999. Higher level phylogeny for the passion-vine butterflies (Nymphalidae, Heliconiinae) based on early stage and adult morphology. Zoo. J. Linn. Soc. 127: 277-344.
Sheppard, P.M., Turner, J.R.G., Brown, K.S., Benson, W.W.,Singer, M.C. 1985. Genetics and the evolution of Muellerian mimicry in Heliconius butterflies. Phil. Trans. R. Soc. Lond. B 308, 433-613.
Turner, JRG. 1967. Goddess changes sex, or the gender game. Syst. Zool. 16: 349-350.
Turner JRG. 1976. Adaptive radiation and convergence in subdivisions of the butterfly genus Heliconius (Lepidoptera: Nymphalidae). Zool. J. Linn. Soc. 58: 297-308.
Title Illustrations

| Scientific Name | Agraulis vanillae |
|---|---|
| Location | Mississippi, USA |
| Specimen Condition | Live Specimen |
| Behavior | Feeding on it's primary host plant lantana. |
| Source | Gulf Fritillary (Agraulis vanillae) |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-NoDerivs License - Version 2.0.
|
| Copyright | © 2003 Jimmy Smith |
| Scientific Name | Dryadula phaetusa |
|---|---|
| Location | captive at The Butterfly Place, Westford, Massachusetts, USA |
| Specimen Condition | Live Specimen |
| Source | The_Butterfly_Place_20 |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2006 Steven Erat |
| Scientific Name | Heliconius sara |
|---|---|
| Location | Portland Zoo, Portland, Oregon, USA |
| Specimen Condition | Live Specimen |
| Source | Small Blue Grecian Butterfly |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution License - Version 2.0. This media file is licensed under the Creative Commons Attribution License - Version 2.0.
|
| Copyright | © 2006 Stuart Seeger |
About This Page
Margarita Beltrán

University of Cambridge, Cambridge, UK
Chris Jiggins

University of Cambridge, Cambridge, UK
Niklas Wahlberg

University of Turku, Finland
Andrew V. Z. Brower

Middle Tennessee State University, Murfreesboro, Tennessee, USA
Correspondence regarding this page should be directed to Margarita Beltrán at
beltran.margarita@gmail.com
, Chris Jiggins at
cj107@cam.ac.uk
, Niklas Wahlberg at
niklas.wahlberg@utu.fi
, and Andrew V. Z. Brower at
abrower@mtsu.edu
Page copyright © 2013 Margarita Beltrán , Chris Jiggins, Niklas Wahlberg, and Andrew V. Z. Brower
 Page: Tree of Life
Heliconiini . Passion-vine Butterflies.
Authored by
Margarita Beltrán, Chris Jiggins, Niklas Wahlberg, and Andrew V. Z. Brower.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Heliconiini . Passion-vine Butterflies.
Authored by
Margarita Beltrán, Chris Jiggins, Niklas Wahlberg, and Andrew V. Z. Brower.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial-ShareAlike License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 25 September 2006
- Content changed 19 May 2013
Citing this page:
Beltrán, Margarita, Chris Jiggins, Niklas Wahlberg, and Andrew V. Z. Brower. 2013. Heliconiini . Passion-vine Butterflies. Version 19 May 2013. http://tolweb.org/Heliconiini/70208/2013.05.19 in The Tree of Life Web Project, http://tolweb.org/












 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site