Fungi
Eumycota: mushrooms, sac fungi, yeast, molds, rusts, smuts, etc.
Meredith Blackwell, Rytas Vilgalys, Timothy Y. James, and John W. Taylor


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxPhylogeny modified from James et al., 2006a, 2006b; Liu et al., 2006; Seif et al., 2005; Steenkamp et al., 2006.
Introduction
The organisms of the fungal lineage include mushrooms, rusts, smuts, puffballs, truffles, morels, molds, and yeasts, as well as many less well-known organisms (Alexopoulos et al., 1996). More than 70,000 species of fungi have been described; however, some estimates of total numbers suggest that 1.5 million species may exist (Hawksworth, 1991; Hawksworth et al., 1995).
As the sister group of animals and part of the eukaryotic crown group that radiated about a billion years ago, the fungi constitute an independent group equal in rank to that of plants and animals. They share with animals the ability to export hydrolytic enzymes that break down biopolymers, which can be absorbed for nutrition. Rather than requiring a stomach to accomplish digestion, fungi live in their own food supply and simply grow into new food as the local environment becomes nutrient depleted.
Most biologists have seen dense filamentous fungal colonies growing on rich nutrient agar plates, but in nature the filaments can be much longer and the colonies less dense. When one of the filaments contacts a food supply, the entire colony mobilizes and reallocates resources to exploit the new food. Should all food become depleted, sporulation is triggered. Although the fungal filaments and spores are microscopic, the colony can be very large with individuals of some species rivaling the mass of the largest animals or plants.

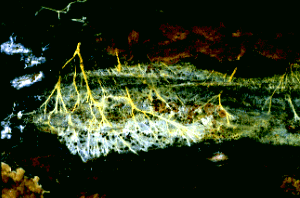
Figure 1: Hyphae of a wood-decaying fungus found growing on the underside of a fallen log. The metabolically active hyphae have secreted droplets on their surfaces. Copyright © M. Blackwell 1996.
Prior to mating in sexual reproduction, individual fungi communicate with other individuals chemically via pheromones. In every phylum at least one pheromone has been characterized, and they range from sesquiterpines and derivatives of the carotenoid pathway in chytridiomycetes and zygomycetes to oligopeptides in ascomycetes and basidiomycetes.
Within their varied natural habitats fungi usually are the primary decomposer organisms present. Many species are free-living saprobes (users of carbon fixed by other organisms) in woody substrates, soils, leaf litter, dead animals, and animal exudates. The large cavities eaten out of living trees by wood-decaying fungi provide nest holes for a variety of animals, and extinction of the ivory billed woodpecker was due in large part to loss, through human activity, of nesting trees in bottom land hardwoods. In some low nitrogen environments several independent groups of fungi have adaptations such as nooses and sticky knobs with which to trap and degrade nematodes and other small animals. A number of references on fungal ecology are available (Carroll and Wicklow, 1992; Cooke and Whipps, 1993; Dix and Webster, 1995).
However, many other fungi are biotrophs, and in this role a number of successful groups form symbiotic associations with plants (including algae), animals (especially arthropods), and prokaryotes. Examples are lichens, mycorrhizae, and leaf and stem endophytes. Although lichens may seem infrequent in polluted cities, they can form the dominant vegetation in nordic environments, and there is a better than 80% chance that any plant you find is mycorrhizal. Leaf and stem endophytes are a more recent discovery, and some of these fungi can protect the plants they inhabit from herbivory and even influence flowering and other aspects of plant reproductive biology. Fungi are our most important plant pathogens, and include rusts, smuts, and many ascomycetes such as the agents of Dutch elm disease and chestnut blight. Among the other well known associations are fungal parasites of animals. Humans, for example, may succumb to diseases caused by Pneumocystis (a type of pneumonia that affects individuals with supressed immune systems), Coccidioides (valley fever), Ajellomyces (blastomycosis and histoplasmosis), and Cryptococcus (cryptococcosis) (Kwon-Chung and Bennett, 1992).

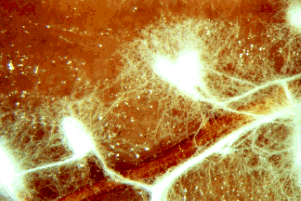
Figure 2: The fluffy white hyphae of the mycorrhizal fungus Rhizopogon rubescens has enveloped the smaller roots of a Virginia pine seedling. Note that some of the mycelium extends out into the surrounding environment. Copyright © J. B. Anderson 1996.


Figure 3: Entomophthora, "destroyer of insects", is the agent of a fungual infection that kills flies. After their death the fungal growth erupts through the fly cuticle, and dispersal by forcible spore discharge is a source of inoculum for infection of new flies. Copyright © G. L. Barron 1996.
Fungal spores may be actively or passively released for dispersal by several effective methods. The air we breathe is filled with spores of species that are air dispersed. These usually are species that produce large numbers of spores, and examples include many species pathogenic on agricultural crops and trees. Other species are adapted for dispersal within or on the surfaces of animals (particularly arthropods). Some fungi are rain splash or flowing water dispersed. In a few cases the forcible release of spores is sufficient to serve as the dispersal method as well. The function of some spores is not primarily for dispersal, but to allow the organisms to survive as resistant cells during periods when the conditions of the environment are not conducive to growth.
Fungi are vital for their ecosystem functions, some of which we have reviewed in the previous paragraphs. In addition a number of fungi are used in the processing and flavoring of foods (baker's and brewer's yeasts, Penicillia in cheese-making) and in production of antibiotics and organic acids. Other fungi produce secondary metabolites such as aflatoxins that may be potent toxins and carcinogens in food of birds, fish, humans, and other mammals.
A few species are studied as model organisms that can be used to gain knowledge of basic processes such as genetics, physiology, biochemistry, and molecular biology with results that are applicable to many organisms (Taylor et al., 1993). Some of the fungi that have been intensively studied in this way include Saccharomyces cereviseae, Neurospora crassa, and Ustilago maydis.
Most phyla appear to be terrestrial in origin, although all major groups have invaded marine and freshwater habitats. An exception to this generality is the flagellum-bearing phyla Chytridiomycota, Blastocladiomycota, and Neocallimastigomycota (collectively referred to as chytrids), which probably had an aquatic origin. Extant chytrid species also occur in terrestrial environments as plant pathogenic fungi, soil fungi, and even as anaerobic inhabitants of the guts of herbivores such as cows (all Neocallimastigomycota).
Characteristics
Fungi are characterized by non-motile bodies (thalli) constructed of apically elongating walled filaments (hyphae), a life cycle with sexual and asexual reproduction, usually from a common thallus, haploid thalli resulting from zygotic meiosis, and heterotrophic nutrition. Spindle pole bodies, not centrioles, usually are associated with the nuclear envelope during cell division. The characteristic wall components are chitin (beta-1,4-linked homopolymers of N-acetylglucosamine in microcrystalline state) and glucans primarily alpha-glucans (alpha-1,3- and alpha-1,6- linkages) (Griffin, 1994).

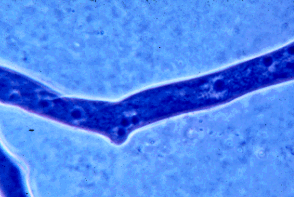
Figure 4: Portion of a hypha of a zygomycete stained with a blue dye to show the many nuclei present. Many other fungi have septations that devide the hyphae into compartments that usually contain one to several nuclei per compartment. Copyright © M. Blackwell 1996.

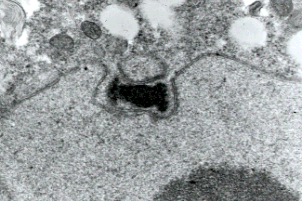
Figure 5: Transmission electron micrograph showing duplicated spindle pole body of a prophase I meiotic nucleus of a basidiomycete Exobasidium. Only chytrids among fungi have centrioles and lack spindle pole bodies. Copyright © Beth Richardson 1996.
Exceptions to this characterization of fungi are well known, and include the following: Most species of chytrids have cells with a single, smooth, posteriorly inserted flagellum at some stage in the life cycle, and centrioles are associated with nuclear division. The life cycles of almost all flagellated fungi are poorly studied. The thalli of Chytridiomycota have been thought to be haploid, but recent population genetic data support diploidy for one species (Morehouse et al. 2003; Morgan et al. 2007). Most members of Blastocladiomycota appear to have sporic meiosis and, therefore, an alternation between haploid and diploid generations. Certain members of Mucoromycotina, Ascomycota, and Basidiomycota may lack hyphal growth during part or all of their life cycles, and, instead, produce budding yeast cells. Most fungal species with yeast growth forms contain only minute amounts of chitin in the walls of the yeast cells. A few species of Ascomycota (Ophiostomataceae) have cellulose in their walls, and certain members of Blastocladiomycota and Entomophthoromycotina lack walls during part of their life cycle (Alexopoulos et al., 1996).
Fossil Record
Based on the available fossil record, fungi are presumed to have been present in Late Proterozoic (900-570 mya). Terrestrial forms of purported ascomycetes are reported in associations with microarthropods in the Silurian Period (438-408 mya) (Sherwood-Pike and Gray, 1985). Fossil hyphae in association with wood decay and fossil chytrids and Glomales-Endogonales representatives associated with plants of the Rhynie Chert are reported from the Devonian Period (408-360 mya) (Hass et al., 1994; Remy et al., 1994a, 1994b; Taylor et al., 1994a, 1995b). Fungal fossil diversity increased throughout the Paleozoic Era (Taylor et al., 1994b) with all modern classes reported in the Pennsylvanian Epoch (320-286 mya).
A first attempt to match molecular data on fungal phylogeny to the geological record shows general agreement, but does point out some conflicts between the two types of data (Berbee and Taylor 1993).
Biogeography
Wherever adequate moisture, temperature, and organic substrates are available, fungi are present. Although we normally think of fungi as growing in warm, moist forests, many species occur in habitats that are cold, periodically arid, or otherwise seemingly inhospitable. It is important to recognize that optimum conditions for growth and reproduction vary widely with fungal species. Diversity of most groups of fungi tends to increase in tropical regions, but detailed studies are only in their infancy (Isaac et al., 1993).
Although many saprobic and plant pathogenic species with low substrate specificity and effective dispersal systems have broad distributions, gene flow appears to be restricted in many fungi. For these species large bodies of water such as the Atlantic and Pacific Oceans create barriers to gene exchange. Some distributions are limited by substrate availability, and dramatic examples come from parasites of Gondowanan plants; one of these is the Southern Hemisphere distribution of the ascomycete Cyttaria, corresponding with part of the distribution of its host plant Nothofagus. The fossil record shows that fungi were present in Antarctica, as is the case for other organisms with Gondwanan distributions. Arthropod associates also may show distributions throughout part or all of a host range, and some fungal species (ex. wood wasp associates) occur outside the range of the associated arthropod.
Notable Fungi
- The largest known basidioma (mushroom or fruiting body) was that of a Rigidioporus ulmarius (Agaricomycetes), hidden-away in a shady corner of the Royal Botanic Gardens, Kew, Richmond, Surrey, England. This fruiting body was mentioned in the Guinness Book of World Records (Matthews, 1994). At the beginning of every New Year the Annual Mensuration Ceremony of the fruiting body took place, and on 19 January 1996 it had increased to 170 cm maximum length (up from 159 in 1995) and 146 cm maximum width (up from 140 in 1995). It also grew 4 cm taller from the soil level, measuring 54 cm. The weight of the fruiting body was estimated to be 284 kg (625 pounds)! Amid rumors of its destruction, Dr. Brian M. Spooner, Head of Mycology, Royal Botanic Gardens, has brought us up to date on the fate of the record specimen. Unfortunately, the basidioma began to rot at the edges a few years ago, likely because the hyphal body of the fungus digested away its elm root substrate, reminding us that a fungus needs a good dispersal system to escape the substrate that eventually inevitably is destroyed. In the life of the fruiting body many trillions of spores must have been produced, and some of these surely fell on an appropiate substrate to establish a new infection. The final insult to the fruiting body came from a fox that burrowed under one side and caused it to collapse. Other large basidiomata are those of a Canadian puffball almost 9 feet in circumference (over 48 pounds) and a basidiocarp of the sulfur mushroom in England (100 pounds). A previous Guinness Book of World Records record-sized fruiting body of Bridgeoporus nobilissimus, an endangered species of the Pacific Northwest of the United States, is over 160 kg (300 pounds) and may have regained the title of “largest” with the demise of the Kew specimen. This polypore also may do itself in because its great weight is likely to eventually cause it to fall as the mycelium depletes its food source, often the noble fir tree.
- Reproductive structures clearly can be very large, but what about the body of the fungus, which often is hidden from view within the substrate? One fungus body constructed of tubular filaments (hyphae) was brought to our attention when molecular techniques were used to show that it was extensive (37 acres and an estimated blue whale equivalent size of 110 tons). The Michigan fungus clone (Armillaria bulbosa, Agaricomycetes) grew in tree roots and soil. This report drew attention to an even larger fungal clone of Armillaria ostoyae, reported earlier in the state of Washington, which covered over 1,500 acres. Each clone began from the germination of a single spore over a thousand years ago. Although they probably have fragmented and are no longer continuous bodies, such organisms give us cause to think about what constitutes an individual.
- Penicillium chrysogenum (Ascomycota) is known for its production of the antibiotic penicillin. Although other antibiotics are produced by a variety of organisms, penicillin was the first to be developed. In the spring of 1996 a long dried out culture of the original isolate prepared by its discoverer, Sir Alexander Fleming in the late 1920s, was auctioned by Sotheby's of London and sold to a pharmaceutical company for 23,000 pounds. This price is insignificant when one considers the worth of this fungus, not only in sales of penicillin, but in terms of illnesses cured and lives saved. In the past a simple scratch or blister sometimes could result in a fatal infection such as the blister that resulted in the death of John Calvin Coolidge (1918-1924), the son of a U. S. president. However, misuse of penicillin and other antibiotics has resulted in selection of resistant microorganisms, and the threat of untreatable bacterial infections and diseases (for example Staphylococcus aureus and Escherichia coli and tuberculosis and syphilis) is still present in our homes and recreation areas.
- Fungal spores fill the air we breathe. On many days in some localities the number of fungal spores in the air far exceeds the pollen grains. Fungal spores also cause allergies; however, unlike seasonal pollen production, some fungi can produce spores all year long. The largest number of fungal spores ever sampled was over 5.5 million per cubic foot in Wales (Matthews, 1994).
- Basidiomycetes have always attracted a lot of attention because some of them have large basidiocarps, but the realization that all fungi are important in ecosystem function has drawn more attention to microscopic forms as well. For example a report on the secret sex life of a yeast-like ascomycete human pathogen, Coccidioides immitis, made a headline of the New York Times (6 February 1996, p. B7). This fungus causes Valley Fever and is endemic in parts of the southwestern United States. Although no one has been able to observe sexual reproduction in this species, molecular studies show genetic diversity that is best explained by occurrence of sexual reproduction in the life cycle.
- Another yeast-like ascomycete reported in the Dallas Morning News (28 August 1995, p. 8D) lives in the gut of cigar beetles and is essential to the beetle's health. Without the gut fungi to detoxify the plant material of toxins, the beetles would be poisoned. Keep on the lookout for other reports of fascinating fungal feats.
Discussion of Phylogenetic Relationships
The kingdom Fungi is a diverse clade of heterotrophic organisms that shares some characters with animals such as chitinous structures, storage of glycogen, and mitochondrial codon UGA encoding tryptophan. Both animals and fungi have spores or gametes with a single smooth, posteriorly inserted flagellum, but only species of the basal chytrid phyla have retained this primitive character (Barr, 1992; Cavalier-Smith, 1987, 1995). Fungi, animals, and other heterotrophic protist-like organisms such as choanoflagellates and Mesomycetozoea are now considered part of the larger group termed opisthokonts (Cavalier-Smith, 1987) in reference to the posterior flagellum.
The branch uniting the fungi and animals is well-supported based on a number of molecular phylogenetic datasets, including the nuclear small subunit ribosomal RNA gene (Wainwright et al., 1993; Bruns et al. 1993), unique and shared sequence insertions in proteins such as elongation factor 1α (Baldauf and Palmer, 1993), entire mitochondrial genomes (Lang et al., 2002), and concatenated protein-coding genes (Steenkamp et al., 2006).
Prior classification systems of Fungi based primarily on morphology are in need of updating to more accurately reflect phylogenetic relationships as determined by molecular systematics. Molecular characters have been essential for phylogenetic analysis in cases when morphological characters are convergent, reduced, or missing among the taxa considered. This is especially true of species that never reproduce sexually, because characters of sexual reproduction traditionally have been the basis for classification of Fungi. Use of molecular characters allows asexual fungi to be placed among their closest relatives.
Previous classifications placed early-diverging fungal groups (non-Ascomycota or Basidiomycota) into two phyla: Chytridiomycota and Zygomycota. Numerous phylogenetic studies now suggest that neither is monophyletic, and the latest classification scheme includes six phyla and an additional four unplaced subphyla (Hibbett et al., 2007). At present, because of the ancient divergence times between the fungal phyla, the exact phylogenetic relationships are ambiguous. Chytrids appear to be a paraphyletic group at the base of the fungal phylogeny and merely fungal lineages which have retained the character of flagellated spores. Three phyla of flagellated fungi are proposed (Blastocladiomycota, Chytridiomycota, and Neocallimastigomycota; Hibbett et al., 2007) and two chytrid genera Olpidium and Rozella, are of uncertain phylogenetic position (James et al., 2006a, 2006b). These genera are interesting because they are both highly reduced endoparasites (living inside the host cell) whose entire thallus consists of only a spherical body absorbing nutrients from the host material that surrounds it. Rozella appears in an isolated position in the fungal phylogeny as the very earliest lineage to diverge from the rest of the fungi (James et al., 2006a, 2006b). In contrast, Olpidium brassicae appears to have diverged after the majority of chytrids and is more closely related to some zygomycete fungi (James et al., 2006a, 2006b).

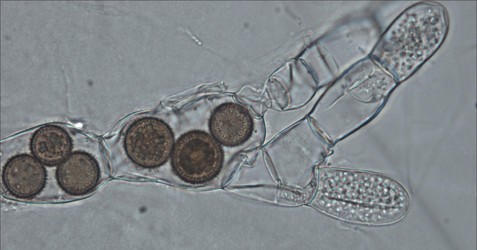
Figure 7: The endoparasitic chytrid Rozella allomycis inside the hyphae of another chytrid Allomyces. Thick spiny spores of the parasite are seen inside some cells while zoospores are produced in other cells. © Timothy Y. James
Fungi with non-septate or irregularly septate hyphae and thick-walled spores were traditionally placed in the phylum Zygomycota. However, evidence for a monophyletic Zygomycota is lacking (Seif et al., 2005), and the deconstruction of the Zygomycota into four unordered subphyla (Entomophthoromycotina, Kickxellomycotina, Mucoromycotina, Zoopagomycotina) has been proposed (Hibbett et al., 2007). The separation of the superficially similar arbuscular mycorrhizal fungi (that lack septa in hyphae but also lack zygospores) into the phylum Glomeromycota has been previously proposed (Schüßler et al., 2001). Whether this phylum is more closely related to the Ascomycota and Basidiomycota lineage or to other zygomycete lineages is controversial (Redecker et al., 2006).
Evidence from shared morphological characters such as regularly septate hyphae and a dikaryotic stage (two separate and different nuclei in a single hyphal segment) in the life cycle usually has been interpreted as support for a close relationship between Basidiomycota and Ascomycota. Numerous phylogenetic studies such as SSU rDNA (Berbee and Taylor, 1992), RNA polymerase genes (Liu et al., 2006), and mitochondrial genome sequencing (Seif et al., 2005) provide strong support for this relationship. A subkingdom termed Dikarya is proposed (Hibbett et al., 2007), creating a division between a highly speciose subkingdom (Dikarya) and the remaining early diverging lineages whose relationships are not precisely known.
Fungal classification is far from static, and even which organisms are actually members of Fungi is changing. For example, the group trichomycetes describes gut inhabitants of arthropods that share similarities with zygomycetes. Molecular phylogenetic studies have demonstrated that two of the four orders of trichomycetes are actually members of the Mesomycetozoea protist group (Benny and O’Donnell, 2000; Cafaro, 2005). Other organisms that were previously considered to be Fungi because of their heterotrophic, mold-like growth forms are now classified as stramenopiles (Oomycota, Hyphochytriomycota, and Labyrinthulomycota) or slime molds (Myxomycota, Plasmodiomycota, Dictyosteliomycota, Acrasiomycota) (Bhattacharya et al., 1992; Leipe et al., 1994; Van der Auwera et al., 1995). More interesting for mycologists are the findings that some species previously considered protozoa are actually Fungi. For example, the species Hyaloraphidium curvatum was assumed to be a green alga that had adopted a heterotrophic lifecycle concomitantly with losing its chloroplast. It is now known to be a chytrid fungus related to Monoblephariomycetes but lacking a flagellated stage (Ustinova et al., 2000). Other examples include the parasitic organisms presumed to be protozoa, such as the cockroach parasite Nepridiophaga (Wylezich et al., 2004) and the Daphnia parasite Polycarum (Johnson et al., 2006) recently demonstrated to be members of the fungal kingdom based on SSU rDNA phylogenies.
The most revolutionary addition to the fungal lineage has occurred with phylogenetic evidence indicating the protist group microsporidia is closely related to Fungi–possibly derived from zygomycetes (Keeling, 2003) or sister to the genus Rozella on the earliest branch in the fungal kingdom (James et al., 2006a). Microsporidia are highly specialized intracellular parasites (primarily of animals) that lack mitochondria but have chitin and trehalose in their spores (similar to Fungi). All molecular studies have shown that microsporidia evolve at an extremely accelerated rate of evolution, making their placement in the Tree of Life difficult. The relationship with fungi is supported by many single and multiple gene phylogenies (e.g., Liu et al., 2006), but an exact placement within the fungi has not received strong support (Keeling and Fast, 2002).
More recently the nuclearid amoebae have been demonstrated to be a sister group to the Fungi with strong support (Steenkamp et al., 2006). This finding is significant because Nuclearia lacks a cell wall and has phagotrophic nutrition in which the food source (such as a bacterium or algal cell) is engulfed wholly, unlike fungi and microsporidia which utilize absorptive nutrition. Further sampling of basal fungal lineages will be needed to determine whether a Nuclearia-like organism was the cenancestor (most recent common ancestor) of Fungi.
References
Alexopoulos, C. J., C. W. Mims, and M. Blackwell. 1996. Introductory Mycology (4th Ed.). John Wiley and Sons, New York, USA. 868p.
Baldauf, S. L., and J. D. Palmer. 1993. Animals and fungi are each other's closest relatives: congruent evidence form multiple proteins. Proceedings of the National Academy of Sciences (USA) 90:11558-11562.
Barr, D. J. S. 1992. Evolution and kingdoms of organisms from the perspective of a mycologist. Mycologia 84:1-11.
Benny G. L. and K. O'Donnell. 2000. Amoebidium parasiticum is a protozoan, not a Trichomycete. Mycologia 92: 1133-1137.
Berbee, M. L., and J. W. Taylor. 1992. Two ascomycete classes based on fruiting-body characters and ribosomal DNA sequence. Molecular Biology and Evolution 9:278-284.
Berbee, M. L., and J. W. Taylor. 1993. Dating the evolutionary radiations of the true fungi. Canadian Journal of Botany 71:1114-1127.
Bhattacharya, D., L. Medlin, P. O. Wainright, E. V. Ariztia, C. Bibeau, S. K. Stickel, and M. L. Sogin. 1992. Algae containing chlorophylls a +c are paraphyletic: molecular evolutionary analysis of the Chromophyta. Evolution 46:801-1817.
Bruns, T. D., T. J. White, and J. W. Taylor. 1991. Fungal molecular systematics. Annual Review of Ecology and Systematics 22:525-564.
Bruns, T. D., R. Vilgalys, S. M. Barns, D. Gonzalez, D. S. Hibbett, D. J. Lane, L. Simon, S. Stickel, T. M. Szaro, W. G. Weisburg, and M. L. Sogin. 1993. Evolutionary relationships within the fungi: analysis of nuclear small subunit rRNA sequences. Molecular Phylogenetics and Evolution 1:231 241.
Cafaro, M. J. 2005. Eccrinales (Trichomycetes) are not fungi, but a clade of protists at the early divergence of animals and fungi. Molecular Phylogenetics and Evolution. 35: 21-34.
Carroll, G.C., and D.T. Wicklow. 1992. The Fungal Community: Its Organization and Role in the Ecosystem. Marcel Deker, Inc., New York.
Cavalier-Smith, T. 1987. The origin of Fungi and Pseudofungi. Pp. 339-353. In: Evolutionary Biology of the Fungi. Eds. A. D. M. Rayner, C. M. Brasier, and D. Moore. Cambridge University Press, Cambridge, United Kingdom.
Cooke, R.C., and J.M. Whipps. 1993. Ecophysiology of Fungi. Blackwell Scientific Pub., London, U.K.
Dix, N.J., and J.W. Webster. 1995. Fungal Ecology. Capman and Hall. London, U.K.
Griffin, D. 1993. Fungal Physiology (2nd Ed.). Wiley-Liss. New York.
Hasegawa, M., T. Hashimoto, J. Adachi, N. Iwabe, and T. Miyata. 1993. Early branchings in the evolution of eukaryotes: ancient divergence of Entamoeba that lacks mitochondria revealed by protein sequence data. Journal of Molecular Evolution 36:380-388.
Hass, H., T. N. Taylor, and W. Remy. 1994. Fungi from the Lower Devonian Rhynie Chert - mycoparasitism. American Journal of Botany 81:29-37.
Hawksworth, D. L. 1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycological Research 95:641-655.
Hawksworth, D. L., P. M. Kirk, B. C. Sutton, and D. N. Pegler. 1995. Ainsworth and Bisby's Dictionary of the Fungi (8th Ed.). CAB International, Wallingford, United Kingdom. 616p.
Hibbett, D. S., M. Binder, J. F. Bischoff, M. Blackwell, P. F. Cannon, O. E. Eriksson, S. Huhndorf, T. James, P. M. Kirk, R. Lücking, T. Lumbsch, F. Lutzoni, P. B. Matheny, D. J. Mclaughlin, M. J. Powell, S. Redhead, C. L. Schoch, J. W. Spatafora, J. A. Stalpers, R. Vilgalys, M. C. Aime, A. Aptroot, R. Bauer, D. Begerow, G. L. Benny, L. A. Castlebury, P. W. Crous, Y.-C. Dai, W. Gams, D. M. Geiser, G. W. Griffith, C. Gueidan, D. L. Hawksworth, G. Hestmark, K. Hosaka, R. A. Humber, K. Hyde, J. E. Ironside, U. Kõljalg, C. P. Kurtzman, K.-H. Larsson, R. Lichtwardt, J. Longcore, J. Miądlikowska, A. Miller, J.-M. Moncalvo, S. Mozley-Standridge, F. Oberwinkler, E. Parmasto, V. Reeb, J. D. Rogers, C. Roux, L. Ryvarden, J. P. Sampaio, A. Schüßler, J. Sugiyama, R. G. Thorn, L. Tibell, W. A. Untereiner, C. Walker, Z. Wang, A. Weir, M. Weiß, M. M. White, K. Winka, Y.-J. Yao, and N. Zhang. 2007. A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509-547.
Isaac, S., J. C. Frankland, R. Watling, and A. J. S. Whalley. 1993. Aspects of Tropical Mycology. Cambridge University Press, Cambridge, U.K.
James, T. Y., F. Kauff, C. Schoch, P. B. Matheny, V. Hofstetter, C. Cox, G. Celio, C. Gueidan, E. Fraker, J. Miadlikowska, H. T. Lumbsch, A. Rauhut, V. Reeb, A. E. Arnold, A. Amtoft, J. E. Stajich, K. Hosaka, G.-H. Sung, D. Johnson, B. O’Rourke, M. Crockett, M. Binder, J. M. Curtis, J. C. Slot, Z. Wang, A. W. Wilson, A. Schüßler, J. E. Longcore, K. O’Donnell, S. Mozley-Standridge, D. Porter, P. M. Letcher, M. J. Powell, J. W. Taylor, M. M. White, G. W. Griffith, D. R. Davies, R. A. Humber, J. B. Morton, J. Sugiyama, A. Y. Rossman, J. D. Rogers, D. H. Pfister, D. Hewitt, K. Hansen, S. Hambleton, R. A. Shoemaker, J. Kohlmeyer, B. Volkmann-Kohlmeyer, R. A. Spotts, M. Serdani, P. W. Crous, K. W. Hughes, K. Matsuura, E. Langer, G. Langer, W. A. Untereiner, R. Lücking, B. Büdel, D. M. Geiser, A. Aptroot, P. Diederich, I. Schmitt, M. Schultz, R. Yahr, D. Hibbett, F Lutzoni, D. McLaughlin, J. Spatafora, and R. Vilgalys. 2006a. Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 443:818-822.
James, T. Y., P. M. Letcher, J. E. Longcore, S. E. Mozley-Standridge, D. Porter, M. J. Powell, G. W. Griffith, and R. Vilgalys. 2006b. A molecular phylogeny of the flagellated Fungi (Chytridiomycota) and a proposal for a new phylum (Blastocladiomycota). Mycologia 98: 860-871.
Johnson, P. T. J., J. E. Longcore, D. E. Stanton, R. B. Carnegie, J. D. Shields, and E. R. Preu. 2006. Chytrid infections of Daphnia pulicaria: development, ecology, pathology and phylogeny of Polycaryum laeve. Freshwater Biology 51: 634-648.
Keeling, P. J. 2003. Congruent evidence from alpha-tubulin and beta-tubulin gene phylogenies for a zygomycete origin of microsporidia. Fungal Genetics and Biology 38: 298-309.
Keeling, P. J., and N. M. Fast. 2002. Microsporidia: biology and evolution of highly reduced intracellular parasites. Annual Review of Microbiology 56: 93-116.
Kwon-Chung, K.J., and J.E. Bennett. 1992. Medical Mycology. Lea and Febiger, Philadelphia.
Lang, B. F., C. O'Kelly, T. Nerad, M. W. Gray, and G. Burger. 2002. Current Biology 12: 1773-1778.
Leipe, D. D., P. O. Wainright, J. H. Gunderson, D. Porter, D. J. Patterson, F. Valois, S. Himmerich, and M. L. Sogin. 1994. The straminopiles from a molecular perspective: 16S-like rRNA sequences from Labyrinthula minuta and Cafeteria roenbergensis. Phycologia 33:369-377.
Liu, Y., M. C. Hodson, and B. D. Hall. 2006. Loss of the flagellum happened only once in the fungal lineage: phylogenetic structure of kingdom Fungi inferred from RNA polymerase II subunit genes. BMC Evolutionary Biology 6:74.
Matthews, P. (Ed.). 1994. Guinness Book of Records. Bantum Books, New York. 819p.
Morehouse, E. A., T. Y. James, A. R. D. Ganley, R. Vilgalys, L. Berger, P. J. Murphy, and J. E. Longcore. 2003. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Molecular Ecology 12:395-403.
Morgan, J. A. T., V. T. Vredenburg, L. J. Rachowicz, R. A. Knapp, M. J. Stice, T. Tunstall, R. E. Bingham, J. M. Parker, J. E. Longcore, C. Moritz, C. J. Briggs, and J. W. Taylor. 2007. Population genetics of the frog killing fungus Batrachochytrium dendrobatidis. Proceedings of the National Academy of Sciences 104:13845-13850.
Nagahama, T., H. Sato, M. Shimazu, and J. Sugiyama. 1995. Phylogenetic divergence of the entomophthoralean fungi: evidence from nuclear 18S ribosomal RNA gene sequences. Mycologia 87:203-209.
Redecker, D., and P. Raab. 2006. Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): recent developments and new gene markers. Mycologia 98: 885-895.
Remy, W., T. N. Taylor, and H. Hass. 1994a. Early Devonian fungi - a blastocladalean fungus with sexual reproduction. American Journal of Botany 81:690-702.
Remy, W., T. N. Taylor, H. Hass, and H. Kerp. 1994b. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proceedings of the National Academy of Sciences (USA) 91:11841-11843.
Rodrigo, A. G., P. R. Bergquist, and P. I. Bergquist. 1994. Inadequate support for an evolutionary link between the metazoa and the fungi. Systematic Biology 43:578-584.
Schüßler, A., D. Schwarzott, and C. Walker. 2001. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycological Research. 105: 1413-1421.
Seif, E., J. Leigh, Y. Liu, I. Roewer, L. Forget, and B. F. Lang. 2005. Comparative mitochondrial genomics in zygomycetes: bacteria-like RNase P RNAs, mobile elements and a close source of the group I intron invasion in angiosperms. Nucleic Acids Research 33:734-744.
Sherwood-Pike, M. A., and J. Gray. 1985. Silurian fungal remains: probable records of the class Ascomycota. Lethaia 18:1-20.
Sidow, A., and W. K. Thomas. 1994. A molecular evolutionary framework for eukaryotic model organisms. Current Biology 4:596-603.
Steenkamp, E. T., J. Wright, and S. L. Baldauf. 2006. The protistan origins of animals and fungi. Mol. Biol. Evol. 23:93-106.
Taylor, J. W., B. Bowman, M. L. Berbee, and T. J. White. 1993. Fungal model organisms: phylogenetics of Saccharomyces, Aspergillus and Neurospora.. Systematic Biology 42:440-457.
Taylor, T. N., W. Remy, H. Hass 1994a. Allomyces in the Devonian. Nature 367:601-601.
Taylor, T. N., J. Galtier, B. J. Axsmith. 1994b. Fungi from the Lower Carboniferous of central France. Review of Palaeobotany and Palynology 83:253-260.
Taylor, T. N., W. Remy, H. Hass, H. Kerp. 1995a. Fossil arbuscular mycorrhizae from the Early Devonian. Mycologia 87:560-573.
Ustinova, I., L. Krienitz and V. A. R. Huss. 2000. Hyaloraphidium curvatum is not a green alga, but a lower fungus; Amobedium parasiticum is not a fungus, but a member of the DRIPs. Protist 151: 253-262.
Van der Auwera, G., R. De Baere, Y. Van de Peer, P. De Rijk, I. Van den Broeck, and R. De Wachter. 1995. The phylogeny of Hyphochytriomycota as deduced from ribosomal RNA sequences of Hyphochytrium catenoides. Molecular Biology and Evolution 12:671-678.
Wainright, P. O., G. Hinkle, M. L. Sogin, and S. K. Stickel. 1993. Monophyletic origins of the Metazoa: an evolutionary link with fungi. Science 260:340-342.
Wylezich, C., R. Radek, and M. Schlegel. 2004. Phylogenetic analysis of the 18S rRNA identifies the parasitic protist Nephridiophaga blattellae (Nephridiophagidae) as a representative of the Zygomycota (Fungi). Denisia 13: 435-442.
Information on the Internet
Popular Sites
- MykoWeb. WWW pages devoted to the science of mycology and the hobby of mushrooming.
- Mycological Society of San Francisco. North America's largest local amateur mycological association.
- Mushroom Observer. The purpose of this site is to record observations about mushrooms, help people identify mushrooms they aren't familiar with, and expand the community around the scientific exploration of mushrooms (mycology).
- The Fungal Jungal. To further educate people about fungi, edible and otherwise, To encourage sustainable and responsible mushroom harvest, and preserve mushroom habitat.
- Tom Volk's Fungi.
- Dave Fischer's North American Mushroom Basics.
- MushroomExpert.Com.
- Forest Fungi.
- Pilze, Pilze, Pilze. In deutsch.
- Westfälischen Pilzbriefe. In deutsch.
- Micologi Associati. Nell'italiano.
Directories, Databases & Collections
- The WWW Virtual Library: Mycology. A well indexed entrance to almost all mycology and fungal biology resources on the Internet.
- Mycology.Net. An internet site containing information about diversity of fungi.
- Mycorrhiza Information Exchange.
- Mycology Online. A WWW resource of clinically significant mycological information.
- Yahoo Mycology.
- Mycologists Online. World-wide directory for mycology and lichenology.
- Fungal Databases. Systematic Botany and Mycology Laboratory. Agricultural Research Service. United States Department of Agriculture. Beltsville, Maryland, USA.
- The Fifth Kingdom online. A mycological encyclopedia.
- Mycological and Lichenological Collection Catalogs. UCMP Berkeley.
- University of Alberta Microfungus Collection & Herbarium (UAMH).
- University of Michigan Fungus Collection.
- Mycological Herbarium. The Natural History Museums and Botanical Garden, University of Oslo.
- Herbarium Mycologicum. National Botanic Garden of Belgium.
- Index Fungorum. Names of fungi.
- Centraalbureau voor Schimmelcultures (CBS). Fungal Biodiversity Center - Utrecht, The Netherlands.
- Fungal Genetics Stock Center.
- Canadian Collection of Fungal Cultures.
Images
- Treasures from the Kingdom of Fungi. Taylor Lockwood's mushroom photography.
- Fungi Images on the Net.
- Fungi, Moulds and Lichens. BioImages: The Virtual Field-Guide (UK).
- George Barron's Website on Mushrooms and other Fungi.
- Eileen's Mushroom Mania.
- Nathan's Fungi Page.
- Pamela's Mushrooms.
Research Labs & Projects
- Deep Hypha. NSF-funded Research Coordination Network (RCN) that is focused on developing robust phylogenetic hypotheses for the deep branches within Kingdom Fungi and enhanced research and educational tools in fungal systematics.
- AFTOL: Assembling the Fungal Tree of Life. Collaborative research in fungal phylogenetics.
- Systematic Botany and Mycology Laboratory. Agricultural Research Service. United States Department of Agriculture. Beltsville, Maryland, USA.
- Forest Mycology and Mycorrhiza Research Team. Forestry Sciences Laboratory, Corvallis, OR, USA.
- Cornell Center for Fungal Biology (CCFB).
- IUCN SSC Fungi Specialist Group Website.
- Mycology at Louisiana State University. Meredith Blackwell's lab.
- Bruns Lab. University of California at Berkeley. Ecology and evolution of fungi.
- Spatafora Lab. Oregon State University. Systematics and evolutionary biology of fungi.
- Taylor Lab. University of California at Berkeley. Evolutionary relationships of fungi, concentrating on the fungi that cause human disease.
- Thorn Lab. University of Western Ontario. Fungal ecology and systematics.
- Vilgalys Lab. Duke University. Natural history of fungi, including all aspects of their evolutionary biology, population genetics, and systematics.
- MycoSite. University of Oslo, Norway.
- University of Georgia Mycology.
- University of Tennessee Mycology Lab.
- Fungal Mitochondrial Genome Project (FMGP). B. Franz Lang, Université de Montréal.
- Fungimap Australia. A collaborative project between professional and amateur mycologists and naturalists to gather information about the distribution of fungi throughout Australia.
- The Fungi of New Zealand. Manaaki Whenua Landcare Research.
- MycoKey. Thomas Læssøe & Jens H. Petersen, University of Aarhus.
- Comparative Studies on the Macrofungi of China and Eastern North America. Qiuxin Wu & Gregory M. Mueller, The Field Museum, Chicago.
- Survey of Northern Illinois and Indiana Fungi. John F. Murphy & Gregory M. Mueller. The Field Museum, Chicago.
- Macrofungi of Costa Rica. Roy E. Halling & Gregory M. Mueller.
- The Fungi of Kenya.
- Malawi Fungi.
- Moulds. Isolation, Cultivation, Identification. David Malloch, Department of Botany, University of Toronto.
Professional Societies
- The International Mycological Association. A group that represents mycologists and fungal biologists throughout the world.
- British Mycological Society.
- Mycological Society of America.
- Asociación Latinoamericana de Micología.
- Australasian Mycological Society.
- The International Society for Mushroom Science. To further the cultivation of edible (including medicinal) macrofungi.
Title Illustrations

| Scientific Name | Chytridium (Chytridiomycota) |
|---|---|
| Comments | Individual growing on a single pine pollen grain. Successive photos show zoospore release from the sporangium, and the arrow points to a flagellum. |
| Copyright | © 1996 H. Whisler, M. Fuller |
| Scientific Name | Pilobolus crystallinus (Mucoromycotina) |
|---|---|
| Comments | Black sporangium atop swollen sporangiophore. Shortly, the swollen subsporangial vesicle will burst to send the sporangium flying. Herbivores eat the sporangium, and the enclosed mitospores germinate in the dung. The bright yellow carotenoid pigment enables the sporangium to orient to light (phototropism). If you look closely, you can see masses of nematodes on the vesicle; probably herbivore pathogens hoping to hitch a ride. |
| Specimen Condition | Live Specimen |
| Copyright |
© 1996 Meredith Blackwell

|
| Scientific Name | Coprinus comatus |
|---|---|
| Location | North Fork John Day Ranger District, Umatilla National Forest, northeastern Oregon, United States |
| Comments | Closed fruiting bodies |
| Specimen Condition | Live Specimen |
| Source | #0808028 |
| Source Collection | Bugwood Network/Forestry Images |
| Image Use |
 This media file is licensed under the Creative Commons Attribution License - Version 3.0. This media file is licensed under the Creative Commons Attribution License - Version 3.0.
|
| Copyright | © Dave Powell, USDA Forest Service |
| Scientific Name | Sarcoscypha coccinea |
|---|---|
| Location | Archidona, Málaga, Andalucía, Spain |
| Comments | Fruiting body of the scarlet cup fungus. Hundreds of millions of meiospores (ascospores) are discharged from this cup, usually in puffs that produce visible clouds of spores. |
| Specimen Condition | Live Specimen |
| Source | Sarcoscypha Coccinea |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 2.0.
|
| Copyright | © 2002 Coqui |
About This Page
Many thanks to Soren Rosendahl and Atul Batra for scanning photos and David Maddison and Atul Batra for page design advice.
Meredith Blackwell

Louisiana State University, Baton Rouge, Louisiana, USA
Rytas Vilgalys

Duke University, Durham, North Carolina, USA
Timothy Y. James

University of Michigan, Ann Arbor, Michigan, USA
John W. Taylor

University of California, Berkeley, California, USA
Correspondence regarding this page should be directed to Meredith Blackwell at
Page copyright © 2012 Meredith Blackwell, Rytas Vilgalys, Timothy Y. James, and John W. Taylor
All Rights Reserved.
- First online 04 July 1996
- Content changed 30 January 2012
Citing this page:
Blackwell, Meredith, Rytas Vilgalys, Timothy Y. James, and John W. Taylor. 2012. Fungi. Eumycota: mushrooms, sac fungi, yeast, molds, rusts, smuts, etc.. Version 30 January 2012. http://tolweb.org/Fungi/2377/2012.01.30 in The Tree of Life Web Project, http://tolweb.org/








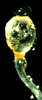




 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site