Bolitaena
Bolitaena pygmaea
Richard E. YoungIntroduction
Bolitaena pygmaea is a small, lower mesopelagic to bathypelagic octopus. It is very similar in appearance to Japetella as small subadults but is distinguished primarily by the smaller eyes that are set on longer optic stalks.
Brief diagnosis:
A bolitaenid ...
- with a ligula on the hectocotylus.
- with small eyes on long optic stalks.
Characteristics
- Arms
- Left arm III hectocotylized in males with an elongate ligula. Third right arm sexually dimorphic with 1-3 greatly enlarged suckers in males.
- Eyes
- Eyes well removed from brain on long optic stalks but within head.
- Eyes small compared to Japetella of comprable size.
Comments
The distinctive hectocotylus makes identification of nearly mature males easy. Unfortunately these males are rarely seen. As a result, separation of this species from Japetella diaphana depends largely on the differences in eye size and length of the optic stalks.
Life history
Near sexual maturity, iridescence of the digestive gland and eyes is lost; pigmentation greatly increases in females, and arms become relatively longer. In addition, the posterior salivary glands of males become greatly enlarged (Voight, 1995). Females develop a yellow ring-shaped light organ around the mouth. Gravid females and nearly mature males (mature males are unknown) are found at the lower end of the vertical distribution range near 1200-1400 m. Brooding females, however, are found near 800 m off Hawaii.


Figure. Oral view of the brachial crown of B. pygmaea showing photophore, mature female, Northwest Atlantic. Photograph by M. Vecchione.
Hatchlings have a ML of about 2 mm and eggs are about the same length (Young, 1972). The early paralarvae of Bolitaena pygmaea have an abundance of chromatophores that easily separates them from Japetella diaphana if specimens are intact which they usually are not. The optic lobe of the brain is distinctly separated from the brain by a short optic stalk even in the paralarva. In Japetella diaphana paralarvae no such separation is apparent.

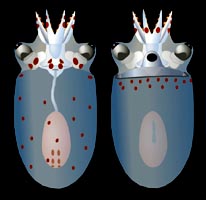
Figure. Dorsal and ventral views of a paralarva of B. pygmaea, 3.9 mm ML. Drawing modified from Hochberg, et al. (1992).
The life history appears to be very similar to that of Japetella diaphana. Present data suggest the following scenario:
Females, brooding clusters of interconnected eggs, float at a depth of around 800 m where downwelling sunlight is too low to reveal their presence. This is about as close as they can get to the surface in Hawaiian waters under this visibility constraint. Females do not feed while brooding and apparently die after the young hatch (Young, 1972). At a temperature of 4-5°C, brooding is expected to take at least several months. Hatchlings either swim upward to about 200 m depth where food presumably is more plentiful or are carried there by the female at the time of hatching. Young Bolitaena descend into mesopelagic depths as they grow, but the size at which they descend is variable (ca. 5-15 mm ML). At maturity the octopus descends to depths of about 1400 m where males probably first attract females then females attract males. Secretions from the enlarged posterior salivary glands of males could secrete pheromones to attract females (for an alternate view of the function of the enlarged salivary glands, see Voight, 1995). Presumably, as the female draws close and considers conditions safe, she will signal the male with the oral light organ and mating will occur.
Distribution
Vertical distribution
In waters off Hawaii, Young (1978) found the distribution pattern shown on the right. Small octopods (ca. 5-15 mm ML) were found near 150-200 m depth or mostly below 600 m. One gravid female was captured at 1400 m. Two nearly mature males were taken at 1200 and 1425 m. Four brooding females were taken between 800 and 900 m.

Figure. Vertical distribution of B. pygmaea off Hawaii. Bars - Fishing range of opening/closing trawl; absence of bar indicates capture is from an open trawl. Circles- Modal depth of the trawl. Open yellow circles- Day captures. Filled blue circles- Night captures. Red dots with yellow cross-bars- Brooding females, day captures. Red dots with blue cross-bars- Brooding females, night capture. Red dot with green ring- Gravid female. Chart redrawn from Young, 1978.
Geographical distribution
This is a tropical-subtropical species found worldwide (Thore, 1949; Nesis, 1982).
References
Chun, C. 1910. Die Cephalopoden. Oegopsida. Wissenschaftliche Ergebnisse der Deutschen Tiefsee Expedition auf dem Dampfer "Valdivia" 1898-1899, 18(1):1-401.
Hochberg, F. G., M. Nixon and R. B. Toll. 1992. Order Octopoda Leach, 1818. In: Sweeney, M. J., C. F. E. Roper, K. M. Mangold, M. R. Clarke and S. v. Boletzky (eds.) "Larval" and juvenile cephalopods: A manual for their identification. Smithson. Contr. Zool., 513:1-282.
Robison, B. and R.E. Young. 1981. Bioluminescence in pelagic octopods. Pac. Sci., 35:39-44.
Thore, S. 1949. Investigations on the "Dana" Octopoda. Dana-Report No. 33: 1-85.
Voight, J. R. 1995. Sexual dimorphism and niche divergence in a midwater octopod (Cephalopoda: Bolitaenidae). Biol. Bull., 189:
Young, R. E. 1972. Brooding in a bathypelagic octopus. Pacific Science, 26: 400-404.
Young, R. E. 1978. Vertical distribution and photosensitive vesicles of pelagic cephalopods from Hawaiian waters. Fish. Bull. 76: 583-615.
Title Illustrations

| Scientific Name | Bolitaena pygmaea |
|---|---|
| Location | off Hawaii |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0. This media file is licensed under the Creative Commons Attribution-NonCommercial License - Version 3.0.
|
| Copyright |
© 1999

|
About This Page

University of Hawaii, Honolulu, HI, USA
Page copyright © 2017
 Page: Tree of Life
Bolitaena . Bolitaena pygmaea .
Authored by
Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Bolitaena . Bolitaena pygmaea .
Authored by
Richard E. Young.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 11 October 2015
Citing this page:
Young, Richard E. 2015. Bolitaena . Bolitaena pygmaea . Version 11 October 2015. http://tolweb.org/Bolitaena_pygmaea/20223/2015.10.11 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site