Aleochara
Christian Maus, Klaus Peschke, and James S. Ashe (1947-2005)- Aleochara (Heterochara)
- Aleochara (Aleochara s. str.)
- Aleochara (Aidochara)
- Aleochara (Euryodma)
- Aleochara (Ceranota)
- Aleochara (Emplenota)
- Aleochara (Triochara)
- Aleochara (Maseochara)
- Aleochara (Echochara)
- Aleochara (Calochara)
- Aleochara (Mesochara)
- Aleochara (Xenochara s. l.)
- Aleochara (Rheochara)
- Aleochara (Polystomota)
- Aleochara (Coprochara)
- Aleochara (Megalogastria)
Introduction
The genus Aleochara comprises approximately 400 species distributed throughout the World (except Antarctica). Because of the parasitic habits and the economic importance of some Aleochara species, the biology of members of this genus is comparatively well known. In contrast, taxonomy at the subgeneric level and the phylogeny are currently poorly resolved.
Characteristics
The genus Aleochara is poorly defined, and little is known about the phylogenetic relationships between the genera within the the subtribe Aleocharini. In fact, some of the currently stated genera might actually need to be included with Aleochara. Therefore, no autapomorphic characteristics of the genus Aleochara can be given.
The body shape of most Aleochara species is more or less broad, stout and robust, fusiform or cylindrical, only few species are rather slender and narrow. The pro-and mesotibiae bear spines. A characteristic of great importance in phylogenetic classification of Aleochara species is the presence or the lack of a mesosternal carina: the former state of the characteristic is considered to be apomorphic, the latter plesiomorphic (KLIMASZEWSKI 1984).
A further characteristic of Aleochara could be the parasitoid behaviour (see "Bionomics"). However, since the development of the species in other Aleocharini genera (and even of the Hoplandriini, the probable sister group of the Aleocharini) is not known, and the presence of at least one non-parasitice species within Aleochara, the possibility that the parasitoid habit is an autapomorphy of Aleochara remains to be confirmed. Species range in size from about 1.5 to 13.0 mm, though the vast majority of the species is between 3 and 8 mm. There are often conspicuous infraspecific size differences (e.g. 2.0-10.0 mm in A. salsipotens). Such dramatic size variation within a species is related to the parasitic development, since the body size of the beetles depends on the size of the host puparia.
Identification Guides
The North American species were revised by KLIMASZEWSKI (1984) (Supplements: KLIMASZEWSKI 1985, KLIMASZEWSKI & BLUME 1986, KLIMASZEWSKI & CERVENKA 1986, KLIMASZEWSKI & GENIER 1987, KLIMASZEWSKI et al. 1990). The European literature is diverse. A selection of the most important European identification guides includes current keys for the Aleochara species of Central Europe (LIKOVSKY 1974, supplement by LOHSE 1989), Great Britain (WELCH 1997) and Scandinavia (PALM 1972, and drawings of genitalia by STRAND & VIK 1968). The Southern African species can be identified with the keys of KLIMASZEWSKI & JANSEN (1993, 1994a, b, c), and the New Zealand species recently have been revised by KLIMASZEWSKI & CROSBY (1997).
Bionomics
Most members of the genus Aleochara live in animal droppings, carrion or decaying plant material. Some species are specialized inquilines of the nests and burrows of certain mammals and birds. A couple of species are associated with rotting mushrooms, and some members of the subgenus Maseochara are found in decaying cacti. Several species are restricted to seashores, where they live under decaying seaweed.
As far as known, the larvae of Aleochara species are solitary ectoparasitoids of cyclorrhaphous Diptera (PESCHKE & FULDNER 1977) (one exception see below). Forty-five species from many taxonomic groups within the genus have been proved to exhibit this life habit (MAUS et al. in prep.). General descriptions of life cycles have been made e.g. by WADSWORTH 1915, KEMNER 1926 and FULDNER 1960. The first instar larva actively searches for a suitable host puparium. It gnaws a small entrance hole through cuticle of the puparium, in many species in a specific area (PESCHKE & FULDNER 1977). In A. curtula, odors from the abdominal spiracles of the host puparium guide the larva (FULDNER 1968). After entering the pupa the Aleochara larva begins to feed on the fly pupa. After completing two molts, the third instar larva of some species leaves the host puparium and pupates in the soil. In other species, the third instar larva pupates in the host puparium (FULDNER & PESCHKE 1977). Only one larva is able to develop in one puparium; there is a severe competition among subsequently arriving larvae (shown for A. bilineata and A. curtula) (FULDNER 1960). There is great variation of body size of adult Aleochara due to different sizes of the host puparia, since most species can develop in puparia of several host species.

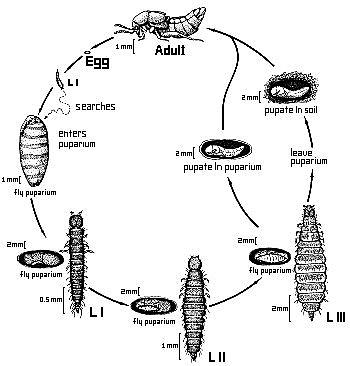
Figure. Generalized life cycle of Aleochara species (Drawing by Sara Taliaferro based on a sketch by Klaus Peschke).
The larval morphology has been described in general e.g. by KEMNER 1926, FULDNER 1960 and WELCH 1964. The first instar larva is campodeoid as in many other Aleocharinae. The second instar larva is eruciform as adaptation to its life within the host puparium. The third instar is also eruciform if it stays within the puparium for pupation, or campodeoid again, if it has a free living period during which it searches for a site for pupation in the soil (PESCHKE & FULDNER 1977). The preimaginal development of Aleochara can be called an hypermetamorphosis, however, it lacks a true sedentary larval stage, as e.g. in the larval development of the Meloidae.
Some Aleochara species are of economic importance as natural enemies of noxious flies (e.g. FABRITIUS 1981, 1987, DREA 1966, JONES 1967, READ 1962, GERSDORF 1962, WRIGHT et al. 1989 and numerous other authors - references compiled in MAUS et al. in prep.). Very important is especially A. (Coprochara) bilineata and some closely related species as parasitoids and predators of anthomyiid vegetable pests (e.g. READ 1962, GERSDORF 1962, JONASSON et al. 1995). A. bilineata is an IOBC-test organism for the investigation of side effects of pesticides (SAMSOE-PETERSEN 1985).
The only species hitherto known, which does has have the parasitoid life cycle typical for Aleochara is A. (Heterochara) clavicornis (PESCHKE et al. 1996). All three larval instars are campodeoid in this species. They feed on decaying meat, fly maggots and also on fly puparia of different stages into which they gnaw large, irregular holes. More than one larva can develop on one prey. This species seems to be a stepping stone in the evolution of the parasitoid life cycle of Aleochara. Phylogenetic and taxonomic implications of life history and larval morphology of this species are currently investigated by two of us (K. PESCHKE, Ch. MAUS).
The reproductive behavior of A. curtula has been extensively investigated. Some selected papers on this topic are PESCHKE 1978, 1990, GACK & PESCHKE 1994.
Subgeneric Classification
The subgeneric classification within the genus Aleochara is not uniform in the literature. At present, the classifications of LIKOVSKY (1974) and of KLIMASZEWSKI (1984) are most frequently used. The former classification is largely based on the "classical" subgeneric system of BERNHAUER & SCHEERPELTZ (1926) (and previous authors), slightly modified mainly by including some small subgenera with the collective group Polychara. KLIMASZEWSKI (1984) and KLIMASZEWSKI & JANSEN (1993) included further subgenera (e. g. Isochara, Polychara, and Notiochara; Baryodma was implicitly included by KLIMASZEWSKI & JANSEN 1993) with the subgenus Xenochara s. l. This latter subgenus now comprises nearly all species with carinate mesosternum that do not have other conspicuous apomorphies, and is a large, heterogenous collective group that is probably not monophyletic. Furthermore KLIMASZEWSKI (1984) united species with incomplete mesosternal carina in the subgenus Calochara; most of these species have previously been included with the subgenus Polychara or small other groups (e. g. Oreochara).
Neither classifications is soundly based in phylogenetic principles (see "Discussion of Phylogenetic Relationships"). Since only a complete revision can produce a sufficient system, we have here outlined the current subgeneric classification, with the awareness that it is preliminary and provisional. Currently, investigations on the infrageneric relationships within the genus Aleochara based on both morphological characteristics and DNA sequences are under way by one of us (Ch. Maus).
The subgeneric classification used here largely follows the classification of KLIMASZEWSKI (1984) and KLIMASZEWSKI & JANSEN (1993), to the extent that they treated the subgenera. In contrast to KLIMASZEWSKI & JANSEN (1994a) who implicitly include the subgenus Heterochara with Aleochara s. str., we maintain this group as a distinct subgenus. For those subgenera that have not been treated by KLIMASZEWSKI l.c., we use the classification of LIKOVSKY (1974) and BERNHAUER & SCHEERPELTZ (1926). The subgenus Mesochara is likely to be a synonym of Calochara sensu KLIMASZEWSKI 1984.
The elevation of individual subgenera to generic rank (e.g. Emplenota, Polystomota) based on conspicuous apomorphies, as proposed by LOHSE 1989, ASSING 1995 and other authors, must be rejected on principle. If autapomorphies can only be provided for the taxon that is elevated to generic rank, but not for the remainder of the main genus, this practice results in formation of paraphyletic groups.
Discussion of Phylogenetic Relationships
The phylogenetic relationships within the genus Aleochara are quite unclear. This is mainly due to the lack of an appropriate subgeneric system (see "Subgeneric Classification"). A large part of the subgenera in their current sense (either sensu LIKOVSKY 1974 and BERNHAUER & SCHEERPELTZ 1926 or sensu KLIMASZEWSKI 1984) are not monophyletic groups, so the construction of a cladogram based on these artificial units is currently impossible.
The large subgenus Aleochara s. str. is a very heterogenous unit that includes most of the species without mesosternal carinae that also lack conspicuous apomorphies. The same is true for the very large subgenus Polychara which includes most species with a carinate mesosternum that lack other striking apomorphies, and even more for Xenochara s. l. (sensu KLIMASZEWSKI 1984), an extremly heterogeneous group which includes Polychara and some smaller subgenera of species with carinate mesosternum. Because of their considerable heterogeneity and their lack of autapomorphies, these two complexes, which together comprise the majority of the Aleochara species, are very likely to be paraphyletic. The same is true for Calochara (sensu KLIMASZEWSKI 1984), which is defined by a symplesiomorphy (i.e. an incomplete mesosternal carina), and some other groups. Some subgenera, e. g. Isochara (which is here included with Xenochara s. l.) are based on superficial similarities (e.g. in punctation) and are probably polyphyletic. There are only a few relatively small subgenera which are robustly monophyletic (e. g. Coprochara, Ceranota, Triochara, Emplenota, Polystomota).
The only attempt to construct a phylogenetic system of the subgenera of Aleochara was made by KLIMASZEWSKI (1984). The cladograms provided in his paper are, however, not completely satisfying: Firstly, as discussed above, some of his subgenera are not monophyletic; some of the proposed sister-group relationships are established upon symplesiomorphies (e. g. Xenochara s. l./Calochara). Secondly, only the subgenera occuring in North America are included within his phylogenetic analysis. Thirdly, the phylogenetic value of some characteristics used to construct the cladograms appears to be questionable (at least on subgeneric level), e.g. body shape, length of the maxillary segments.
However, reconstruction of a more robust cladistic hypothesis would require a revision of the subgeneric system of Aleochara based on phylogenetic principles (current investigations, see "Subgeneric Classification").
Emplenota is probably the sister group of the subgenus Triochara. ASSING (1995) points out that there are some distinctive characteristics that both subgenera share. The most phylogenetically important character is presence of ventral processes on the aedeagal median lobe. These processes are unique to members of these two subgenera and are probably a synapomorphy for them. The subgenus Emplenota is not likely to be the sister-group of Maseochara, as proposed by KLIMASZEWSKI 1984, since the subgenus Triochara appears to be much more closely related to the former group (see MAUS & ASHE 1997).
KLIMASZEWSKI 1984 affiliates Echochara (together with Emplenota and Maseochara) to a group of subgenera that he hypothesized to be monophyletic based on the distinct microsculpture of the forebody as synapomorphy. However, distinct microsculpture is present in very different groups within Aleochara (e. g. Polystomota, Emplenota, Coprochara partially, Maseochara), so this characteristic is likely to have developed independently in different evolutionary lineages. Thus, it is questionable that the distinct microsculpture is a synapomorphy of the three subgenera mentioned above, and that these three subgenera actually constitute a monophyletic lineage.
The phylogenetic relationships of Polystomota to other subgenera or species groups within Aleochara have not been investigated. However, Polystomota is probably not closely related to the superficially very similar subgenus Emplenota. These two taxa distinctly differ in some phylogenetically important characteristics (e.g. presence or absence of a mesosternal carina and of ventral processes of the aedeagal median lobe, visibility of the hypomera in lateral view). The external similarity of both subgenera probably results from parallel development of characteristics caused by adaptation to similar environmental conditions (seashore habitat).
Call for Cooperation
A project to unravel the phylogeny of the genus Aleochara is under way at the University of Freiburg, including molecular (DNA-sequences), biochemical (chemotaxonomy with cuticular hydrocarbon patterns and defensive secretion), morphological (adults and larvae), and behavioral data. The authors (K. Peschke, Ch. Maus) would greatly appreciate receiving specimens from anywhere of the world, freshly killed and fixed in absolute ethanol. Live specimens are most welcome; we can send instructions about how to mail live beetles (address see below). We would be also be glad to receive spare specimens (dried) from collections and any information about unpublished host records or unpublished data from ecological studies.
References
Bernhauer, M. & O. Scheerpeltz 1926. Staphylinidae VI. In: Junk, W. & S. Schenkling: Coleopterorum Catalogus. Pars 82. Junk, Berlin. pp. 499-988.
Drea,J. 1966. Studies of Aleochara tristis (Col., Staphylinidae), a natural enemy of the Face Fly. Journal of Economic Entomology 59 (6): 1368-1373.
Fabritius, K. 1981. Ueber das natuerliche Vorkommen und den Wirtskreis von Parasiten synanthroper Fliegen. Zeitschrift fuer angewandte Zoologie 68/2: 174-181.
Fuldner, D. 1960. Beitraege zur Morphologie und Biologie von Aleochara bilineata Gyll. and A. bipustulata (Coleoptera, Staphylinidae). Zeitschrift fuer Morphologie und Oekologie (Tiere) 49: 312-386.
Fuldner, D. 1968. Experimentelle Analyse des Orientierungsverhaltens der Eilarve von Aleochara curtula GOEZE (Coleoptera, Staphylinidae). Zeitschrift fuer vergleichende Physiologie 61: 298-354.
Gack C. & Peschke K. 1994. Spermathecal morphology, sperm transfer and a novel mechanism of sperm displacement in the rove beetle, Aleochara curtula (Coleoptera, Staphylinidae). Zoomorphology 114: 227-237.
Gersdorf, E. (1962): Zur Biologie einiger Arten der Gattung Aleochara Grav. Entomologische Blaetter 58 (3): 178-182.
Jonasson, Th., M. Ahlstroem-Olsson & T.J. Johansen 1995. Aleochara suffusa and A. bilineata (Col: Staphylinidae) as parasitoids of Brassica Root Flies in Northern Norway. Entomophaga 40 (2): 163-167.
Jones, C.M. 1967. Aleochara tristis, a natural enemy of Face Fly. I. Introduction and laboratory rearing. Journal of Economic Entomology 60 (3): 816-817.
Kemner, N.A. 1926. Zur Kenntnis der Staphyliniden-Larven II. Die Lebensweise und die parasitische Entwicklung der echten Aleochariden. Entomologisk Tidskrift 47: 133-170.
Klimaszewski, J. 1984. A revision of the genus Aleochara Gravenhorst of America north of Mexico (Coleoptera: Staphylinidae, Aleocharinae). Memoirs of the Entomological Society of Canada 129: 1-211.
Klimaszewski, J. 1985. Nomenclatorical changes in the genus Aleochara Gravenhorst (Coleoptera: Staphylinidae: Aleocharinae). Coleopterists Bulletin 39: 376.
Klimaszewski, J. & R. Blume 1986. New host records for Aleochara verna Say and Aleochara notula Erichson (Coleoptera: Staphylinidae, Aleocharinae). Coleopterists Bulletin 40: 32.
Klimaszewski, J. & V. Cervenka1986. A revision of the genus Aleochara Gravenhorst of America north of Mexico (Coleoptera: Staphylinidae, Aleocharinae). Supplement 3. New distribution data. Entomological News 97: 119-120.
Klimaszewski, J. & T.K. Crosby 1997. A revision of the New Zealand species of the parasitoid genus Aleochara, with description of four new species (Coleoptera: Staphylinidae). Journal of the Royal Society of New Zealand 27(2): 243-269.
Klimaszewski, J. & F. Genier 1987. A revision of the genus Aleochara Gravenhorst of America north of Mexico (Coleoptera: Staphylinidae, Aleocharinae). Supplement 4. New distribution data and descriptions of two new species. Coleopterists Bulletin 41(3): 241-248.
Klimaszewski, J., J.H. Frank & S.B. Peck 1990. Two new species and a key to the adults of Aleochara of Florida (Coleoptera: Staphylinidae). Florida Entomologist 73(1): 177-185.
Klimaszewski, J. & R. Jansen 1993. Systematics, biology and distribution of Aleochara Gravenhorst from Southern Africa. Part 1: subgenus Xenochara Mulsant & Rey (Coleoptera: Staphylinidae). Annals of the Transvaal Museum 36(7): 53-107.
Klimaszewski, J. & R. Jansen 1994a. Systematics, biology and distribution of Aleochara. Part II: subgenus Aleochara (Coleoptera, Staphylinidae). Journal of African Zoology 108: 93-117.
Klimaszewski, J. & R. Jansen 1994b. Systematics, biology and distribution of Aleochara Gravenhorst from Southern Africa. Part 3: subgenus Coprochara Mulsant & Rey (Coleoptera: Staphylinidae). Annals of the Transvaal Museum 36(10): 147-170.
Klimaszewski, J. & R. Jansen 1994c. Systematics, biology and distribution of Aleochara Gravenhorst. Part 4: subgenus Maseochara Sharp in the Afrotropical region. Journal of African Zoology 108: 163-180
Likovsky, Z. 1974. 237. Gattung: Aleochara Gravenhorst 1802. In: Freude, H., K.W. Harde & G.A. Lohse: Die Käfer Mitteleuropas. Band 5: Staphylinidae II (Hypocyphtinae und Aleocharinae), Pselaphidae. Goecke & Evers, Krefeld: 381 pp.
Lohse, G.A. 1989. 23. Familie Staphylinidae (II) (Aleocharinae). In: Lohse, G.A. & W.H. Lucht: Die Käfer Mitteleuropas. Band 12 (1. Supplementband). Goecke & Evers, Krefeld: 346 pp.
Maus, Ch, B. Mittmann & K. Peschke in prep.. Survey of host records of parasitoid Aleochara species (Coleoptera: Staphylinidae) attacking cyclorrhaphous fly puparia: Review and new investigations.
Palm, Th. 1972. Skalbaggar. Coleoptera. Kortvingar: Fam. Staphylinidae, Underfam. Aleocharinae (Aleuonota-Tinotus). Svensk Insektfauna 9(7): 460 pp.
Peschke, K. 1978. Funktionsmorphologische Untersuchungen zur Kopulation von Aleochara curtula GOEZE (Coleoptera, Staphylinidae). Zoomorphologie 89: 157-184.
Peschke, K. 1990. Chemical traits in sexual selection of the rove beetle, Aleochara curtula (Coleoptera: Staphylinidae). Entomologia Generalis 15:127-132.
Peschke, K. & D. Fuldner 1977. Uebersicht und neue Untersuchungen zur Lebensweise der parasitoiden Aleocharinae (Coleoptera; Staphylinidae). Zoologische Jahrbuecher (Systematik) 104: 242-262.
Peschke, K., B. Mittmann & D. Fuldner (1996) Aleochara clavicornis, a stepping stone in the evolution of parasitoid behavior within the rove beetle genus Aleochara (Coleoptera, Staphylinidae). Proceedings of XXth International Congress of Entomology, Firenze, August 25-31, 1996.
Read, D.C. 1962. Notes on the life history of Aleochara bilineata (Gyll.) (Coloptera: Staphylinidae), and on ist potential value as a control agent for the Cabbage Maggot, Hylemya brassicae (Bouche) (Diptera: Anthomyiidae). Canadian Entomologist 94: 417-424.
Samsoe-Petersen, L. 1985. Laboratory Tests to Investigate the Effects of Pesticides on Two Beneficial Arthropods: a Predatory Mite (Phytoseiulus persimilis) and a Rove Beetle (Aleochara bilineata). Pesticide Science 16: 321-331.
Strand, A. & A. Vik (1968): Die Genitalorgane der nordischen Arten der Gattung Aleochara Grav. (Col., Staphylinidae). Norsk entomologisk Tidskrift 15: 105-110.
Wadsworth, J.T. 1915. On the life history of Aleochara bilineata GYLL., a staphylinid parasite of Chortophila brassicae Bouche. Journal of economic Biology 10: 1-27.
Welch, R.C. 1964. The biology of the genus Aleochara Grav. (Coleoptera, Staphylinidae). Ph. D. Thesis, University of London: 415 pp.
Welch, R.C. 1997. The British species of the genus Aleochara Gravenhorst (Staphylinidae). The Coleopterist 6 (1): 1-45.
Wright, E., P. Mueller & J. Keer 1989. Agents for biological control of novel hosts: assessing an Aleocharine parasitoid of dung-breeding flies. Journal of applied Ecology 26: 453-461.
Title Illustrations

| Scientific Name | Aleochara (s.str.) lata |
|---|---|
| Location | Italy |
| Size | length 6.5 mm |
| Copyright |
© 1997 James S. Ashe (1947-2005)

|
| Scientific Name | Aleochara (Emplenota) pacifica |
|---|---|
| Location | Canada, B.C. |
| Size | length 5.6 mm |
| Copyright |
© 1997 James S. Ashe (1947-2005)

|
| Scientific Name | Aleochara (Xenochara) kamila |
|---|---|
| Location | France |
| Size | length 4.0 mm |
| Copyright |
© 1997 James S. Ashe (1947-2005)

|
About This Page
The drawing of the life cycle of Aleochara on this page is by Sara Taliaferro. Development of this page made possible by National Science Foundation PEET grant DEB 95-21755 to James S. Ashe and a DAAD grant D/97/05475 from the German Government to Christian Maus.
Christian Maus

Bayer CropScience AG, Institute for Ecotoxicology, Monheim, Germany
Klaus Peschke

Universität Freiburg, Germany
James S. Ashe (1947-2005)

University of Kansas, Lawrence, Kansas, USA
Correspondence regarding this page should be directed to Christian Maus at
christian.maus@bayercropscience.com
Page copyright © 1997 Christian Maus , Klaus Peschke, and James S. Ashe (1947-2005)
 Page: Tree of Life
Aleochara.
Authored by
Christian Maus, Klaus Peschke, and James S. Ashe (1947-2005).
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Aleochara.
Authored by
Christian Maus, Klaus Peschke, and James S. Ashe (1947-2005).
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 11 September 1998
Citing this page:
Maus, Christian, Klaus Peschke, and James S. Ashe (1947-2005). 1998. Aleochara. Version 11 September 1998. http://tolweb.org/Aleochara/9878/1998.09.11 in The Tree of Life Web Project, http://tolweb.org/













 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site