Agaricales
Brandon Matheny, Jean-Marc Moncalvo, and Scott A. Redhead


This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxRelationships after Binder, Matheny & Hibbett (unpublished data), Matheny et al. (2006), Binder et al. (2005), Bodensteiner et al. (2004), Larsson et al. (2004), Moncalvo et al. (2000, 2002), Singer (1986), and Kühner (1980).
Inclusive major clade names (e.g., plicaturopsidoid, hygrophoroid) are from Matheny et al. (2006) and are informal. The monophyly of these major clades receive significant support with Bayesian analyses with exception of the pluteoid clade.
Introduction
The Agaricales, or euagarics clade, is a monophyletic group of approximately 8500 mushroom species. As such it forms the largest clade of Agaricomycetes (=Homobasidiomycetes sensu Hibbett and Thorn 2001; Binder et al. 2005) and together with the Boletales and Atheliales it forms the Agaricomycetidae. Species of Agaricales are widespread and diverse on land ranging from desert, grassland, forests, tundra, and shorelines in tropical, temperate, and arctic-alpine habitats. While many Agaricales acquire sustenance by decomposing dead organic matter (as saprotrophs), others parasitize plants or other fungi, and a few capture or parasitize vertebrates or invertebrates. Many groups of Agaricales engage in mutualistic symbioses with the roots of vascular plants where they form ectomycorrhizas (EM), or more rarely associate with unicellular green algae or cyanobacteria as lichens. A few colonize bryophytes. Orchids and mycotrophic plants depend on some Agaricales (Armillaria, Tricholoma), in addition to other groups of Agaricomycetes, for their nutrition. Several clades of Agaricales include ant or termite symbionts or even nematode predators. Species of Armillaria, Athelia pro parte, Moniliophthora, and Mycena are economically devastating plant pathogens, whereas others in the genera Agaricus, Lentinula, Termitomyces, Tricholoma, and Volvariella are highly valuable commercial or collected foods. Psychotropic or hallucinogenic mushrooms of Psilocybe and other genera also occur in the Agaricales, and others, as in some species of Amanita, produce toxins lethal to humans.



A sample of different nutritional modes among Agaricales. Left: Ectomycorrizal (EM) habitat in Karri (Eucalyptus diversicolor) forest in the south-west botanical district of Western Australia. © Brandon Matheny. Center: Attack and consumption of a nematode by Pleurotus (Oyster Mushroom, Pleurotaceae). © Greg Thorn. Right. Termitomyces reticulatus (Lyophyllaceae) fruiting from underground fungus gardens of the termite Odontotermes badius. © Duur Aanen.
Molecular systematic studies of the Agaricales have radically transformed our interpretations of the evolution and classification of gilled mushrooms and their relatives (Hibbett et al. 1997; Moncalvo et al. 2000, 2002; Matheny et al. 2006). The overwhelming majority of species produces fruit bodies with gills (lamellae), but the evolution of gills has arisen numerous times in the Agaricomycetes (Hibbet et al. 1997). Likewise, multiple lineages of “gasteromycetes” (puffballs, bird’s nest fungi, false truffles), species that produce spores in an enclosed fruiting structure, have evolved independently among the Agaricales (Peintner et al. 2001). Studies by Bodensteiner et al. (2004), Larsson et al. (2004), and Binder et al. (2005) have shown that some non-gilled fungi, including reduced or cup-like (cyphelloid) forms and crustose or resupinate forms, share their evolutionary histories with numerous lineages of Agaricales, including some lineages that evolved in aquatic or marine environments (Binder et al. 2001; Hibbett and Binder 2001; Binder et al. 2006). In short, the gross morphology of mushroom fruit bodies is highly plastic and often a poor phylogenetic indicator. These and other studies demonstrated that a broad concept of Agaricales (Singer 1986), including boletes, some polypores, and the genera Russula, Lactarius and their allies, does not form a monophyletic group. Thus, the clade containing predominantly genera and families from the suborder Agaricineae (Singer 1986) was labeled the euagarics clade and represents what we currently regard as the Agaricales (Moncalvo et al. 2002). Most family-level relationships based on morphological characters are artificial, but progress is being made to delimit higher-level monophyletic groups with multiple gene data sets (Matheny 2005; Aime et al. 2005; Hofstetter et al. 2002; Binder et al. 2006). Remarkably, new species and genera continue to be described or placed in the order by molecular phylogenetic analyses.
Characteristics


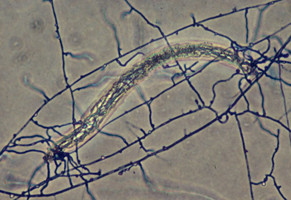
Scanning electron microscopic (SEM) images of basidiospores of Agaricales. Left: Spinose basidiospore of Inocybe calospora (Inocybaceae). © Brandon Matheny. Right: Grooved basidiospores of Clitopilus prunulus (Entolomataceae). © .
No morphological, ecological, or biochemical traits are known that unite the Agaricales despite robust measures of support for its monophyly (Binder and Hibbett 2002; Moncalvo et al. 2002; Matheny et al. 2007). Singer (1986), one of the most influential taxonomic treatments of the order, was unable to provide a set of non-molecular characters unique to all members. Rather, inclusion in the group, in the absence of molecular confirmation, was historically made by the absence of certain characters possessed by other clades of Agaricomycetes. For example, members of the Agaricales sensu Singer were attributed to have non-septate basidia (unlike Auriculariales), lack stichobasidia (unlike some families of Cantharellales), lack trimitic hyphal systems (unlike Polyporales and Hymenochaetales), not possess a spinose hymenophore (unlike Thelephorales), lack variegatic acid type pigments and its derivatives (unlike Boletales), and not have heteromerous trama or a combination of a laticiferous hyphal system with amyloid, ornamented spores (unlike Russulales). No members of the Agaricales produce a yeast-like stage, but many non-mycorrhizal groups may produce conidia or other unique asexual reproductive structures (Walther et al. 2005). The spore bearing surface (hymenophore) is typically lamellate and infrequently tubular (unlike Boletales). However, it is becoming clear that cyphelloid and false truffles have likely evolved from lamellate ancestors, as have some species producing club-shaped (clavarioid) basidiomes. It remains unclear whether the most recent common ancestor of the Agaricales was gilled (Matheny et al. 2006).




Examples of non-gilled fruit body forms in the Agaricales. Left: Clavarioid fruit bodies of Clavulinopsis fusiformis (Clavariaceae s. str.). © Brandon Matheny. Gasteroid fruit bodies of Crucibulum laeve (Nidulariaceae). © Mark Steinmetz. Resupinate fruit bodies of Cylindrobasidium evolvens (Physalacriaceae). © Brandon Matheny.
Despite the absence of morphologically shared derived traits (synapomorphies) for the Agaricales as a whole, some combinations of traits do appear diagnostic for nested lineages within the order. Species with pigmented spore walls, multinucleate spores, and spores with an open-pore type of hilum (an ultrastructural character) (Pegler and Young 1969) appear derived and belong to the agaricoid clade. All these characters, with exception of the hilum-type, may have evolved repeatedly on independent occasions. Nevertheless, it appears reasonable that the most recent common ancestor of the Agaricales was probably a saprotroph or parasite with uninucleate, hyaline spores with a nodulose spore hilum, perhaps similar to early-diverging lineages of the Atheliales and Boletales, two groups most closely related to the Agaricales. The ectomycorrhizal condition, a symbiosis between fungal hyphae and typically roots of vascular plants, appears to have evolved independently at least on eleven occasions in the Agaricales alone without any unamibiguous reversals to a free-living state (Matheny et al. 2006).
Discussion of Phylogenetic Relationships
Members of the earliest lineage of Agaricales, the plicaturopsidoid clade, are not very well known. At present the clade includes a diverse assemblage of taxa composed of resupinate, merulioid, club-like, coralloid, and gilled forms that are saprotrophic or pathogenic. No known EM lineages have yet been confirmed. Larsson et al. (2004) and Binder et al. (2005) identified additional resupinate, club-like, coralloid, and false truffle species at the base of the Agaricales, but these have yet to be integrated in a single cohesive analysis. Unpublished findings, together with results from Matheny et al. (2006), suggest many of these poorly known genera occupy early diverging lineages of Agaricales using a Bayesian method of inference. The diversity of fruitbody morphologies in the group, including species of Podoserpula, Plicaturopsis, Camarophyllopsis, Athelia pro parte, and the Clavariaceae, suggest these traits are highly plastic and unreliable gross phylogenetic markers. Separate 5.8S/25S and 25S only rRNA studies (Larsson et al. 2004; Dentinger & McLaughlin 2006), however, embed the Clavariaceae within derived groups of Agaricales with poor support using the maximum parsimony method. Parsimony analyses of combined protein-coding and ribosomal RNA data sets place the Clavariaceae at the base of the hygrophoroid clade but with weak support, or in a poorly supported grade with Plicaturopsis and allies before the split of the remaining Agaricales. Thus, the phylogenetic position of the Clavariaceae merits more attention.
The plicaturopsidoid clade appears to be the sister group to a crown group of Agaricales, which is dominated by gilled forms. Some of these genera and families of the crown group remain poorly supported by the parsimony bootstrap method despite a recent supermatrix phylogenetic analysis that included up to six nuclear gene regions for 250 taxa (Matheny et al. 2006). Nonetheless, at least five additional inclusive clades of mushrooms were recovered by Bayesian analyses.
Based on these latter results, the preponderance of EM formers appears to have evolved within the agaricoid and tricholomatoid clades, which are sister groups. Only two additional EM lineages are presently identified in the remaining Agaricales, the Amanitaceae and Hygrophorus s. str., but the nutritional mode of many other groups is poorly known (e.g., Cantharocybe, Clavariaceae s. str.). The agaricoid clade contains the dark-spored Agaricales, and all taxa appear to be characterized by multinucleate spores with an open-pore hilum. This group contains many truffle-like species that are EM formers and tend to sporulate below the surface of the ground (hypogeous), as well as the bird’s nest fungi (Nidulariaceae), which are saprotrophs. The tricholomatoid clade includes lineages with white or pink spores and species with diverse ecologies including mycoparasites.
Of ecological interest, no EM taxa are known in the taxonomically diverse marasmioid clade, which is dominated by saprophtrophic white-spored taxa. Reduction of fruit bodies from gilled forms to cupulate forms (cyphelloid) appears to be a morphological tendency in this large inclusive lineage, in which some genera (e.g., Marasmius) are abundant in the tropics (Singer 1986). The hyrophoroid clade (e.g., Pterulaceae and Hygrophoraceae s. lat.) comprises predominantly white-spored taxa (rarely pink) that are also mainly saprotrophic. However, a few pathogens (Typhula spp.) and lichenized lineages (Dictyonema, Lichenomphalia) are placed in this clade, along with the aforementioned EM lineage Hygrophorus. The pluteoid clade is composed of pinkish brown and white-spored taxa of the families Pluteaceae, Macrocystidiaceae, and Limnoperdonaceae. Kühner (1980) presents morphological evidence that suggests a relationship between the Pluteaceae and Macrocystidiaceae, which we accept here and which is also supported by combined rRNA analyses (Matheny et al. 2006). Phylogenetic relationships between the pluteoid clade and families Amanitaceae and Pleurotaceae were not strongly supported by Bayesian or parsimony multigene analyses and require further investigation, hence, these families are shown in unresolved positions.



Gilled fruitbodies. Left: Gliophorus laetus (Hygrophoraceae s. lat.). © Brandon Matheny. Right: Fresh fruitbodies of Coprinus comatus (Agaricaceae). The gills of the specimens on the left are undergoing auto-digestion (deliquescence). © David LaPuma.
Agaricales incertae sedis
At present we have little or no phylogenetic information that sheds light on the relationship of at least 58 genera and clades of gilled, cyphelloid, and resupinate fungi (including four fossil genera) with affinities to the Agaricales. While single gene analyses have confirmed a relationship of many of these groups to the order (Moncalvo et al. 2002, Bodensteiner et al. 2004), their positions within the Agaricales are not clear based on single gene phylogenetic analyses. Future phylogenetic research of the Agaricales should focus taxon sampling among these genera.
Fossil Genera and Age of the Agaricales
The phylogeny and age of the Agaricales, and other fungi, is difficult to reconstruct from the fossil record (Taylor & Osborn 1996). Only four fossil genera of gilled mushrooms are currently accepted (Hibbett et al. 2003), with the oldest of these dating back to the mid-Cretaceous, about 90 million years ago. All are suspected to share affinities with the Agaricales. Fossil ectomycorrhizas, probably of Boletales, have been dated back to the mid-Eocene, about 50 million years ago (LePage et al. 1997). Using a molecular clock, Berbee and Taylor (1993) hypothesized that ectomycorrhizal fungi may have evolved 130 million years ago during the early Cretaceous. However, the age of the origin and diversification of the Agaricales has not been ascertained by molecular clock dating methods. The Agaricales is one of three clades, together with the Atheliales and Boletales, that appear to form one of the more derived groups of Agaricomycetes (Binder et al. 2005; Matheny et al. 2007). If cladogenesis of the sister group to the Agaricales (the Boletales) is used as an indicator (Bruns et al. 1998), then perhaps the Agaricales may have radiated simultaneously, which is estimated between 75 and 135 million years ago. Two recent studies, however, have hypothesized mid- to late-Cretaceous divergences for two genera of dark-spored Agaricales (Geml et al. 2004; Matheny and Bougher 2006), which might suggest an older radiation for the order at large.

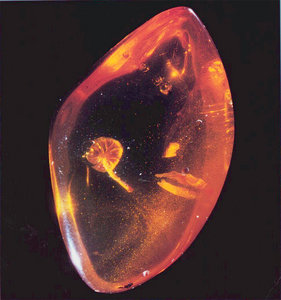
Protomycena electra, a mushroom fossil from Miocene amber in the Dominican Republic.
© David Grimaldi. Image courtesy of David Hibbett.
References
Aime, M. C., and Phillips-Mora, W. 2005. The causal agents of witches’ broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia 97: 1012-1022.
Berbee, M. L., and Taylor, J. W. 1993. Dating the evolutionary radiations of the true fungi. Can. J. Bot. 71: 1114-1127.
Binder, M., and Hibbett, D. S. 2002. Higher-level phylogenetic relationships of Homobasidiomycetes (mushroom-forming fungi) inferred from four rDNA regions. Mol. Phylogenet. Evol. 22: 76-90.
Binder, M., Hibbett, D. S., Larsson, K. H., Larsson, E., Langer, E. and Langer. G. 2005. The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (Homobasidiomycetes). Syst. Biodiversity 3: 113-157.
Binder, M., Hibbett, D. S., and Molitoris, H. P. 2001. Phylogenetic relationships of the marine gasteromycete Nia vibrissa. Mycologia 93: 679-688.
Binder, M., Hibbett, D. S., Wang, Z., and Farnham, W. F. 2006. Evolutionary relationships of Mycaureola dilseae (Agaricales), a basidiomycete pathogen of a subtidal rhodophyte. Am. J. Bot. 93: 547-556.
Bodensteiner, P., Binder, M., Moncalvo, J. M., Agerer, R., and Hibbett, D. S. 2004. Phylogenetic relationships of cyphelloid homobasidiomycetes. Mol. Phylogenetic. Evol. 33: 501-515.
Bruns, T. D., Szaro, T. M., Gardes, M., Cullings, K. W., Pan, J. J., Taylor, D. L., Horton, T. R., Kretzer, A., Garbelotto, M., and Li, Y. 1998. A sequence database for the identification of ectomycorrhizal basidiomycetes by phylogenetic analysis. Mol. Ecol. 7: 257-272.
Dentinger, B. T. M., and McLaughlin, D. J. 2006. Reconstructing the Clavariaceae using nuclear large subunit rDNA sequences and a new genus segregated from Clavaria. Mycologia 98: 746-762.
Geml J., Geiser, D. M. and Royse, D. J. 2004. Molecular evolution of Agaricus species based on ITS and LSU rDNA sequences. Mycol. Progress 3: 157-176.
Hibbett, D. S., Pine, E. M., Langer, E., Langer, G. and Donoghue, M. J. 1997. Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proc. Nat. Acad. Sci. USA 94:12002-12006.
Hibbett, D. S. and Binder, M. 2001. Evolution of marine mushrooms. Biol. Bull. 201: 319-322.
Hibbett, D. S. and Thorn, R. G. 2001. Homobasidiomycetes. Pp. 121-170. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Hibbett, D. S., Binder, M., Wang, Z. and Goldman, Y. 2003. Another fossil agaric from Dominican amber. Mycologia 95: 685-687.
Hofstetter, V., Clémençon, H. Vilgalys, R., and Moncalvo, J. M. 2002. Phylogenetic analyses of the Lyophylleae (Agaricales, Basidiomycetes) based on nuclear and mitochondrial rDNA sequences. Mycol. Res. 106: 1043-1059.
Kühner, R. 1980. Les Hyménomycètes agaricoïdes. Bull Soc Linn Lyon 49: Numéro spécial. 1027 p.
Larsson, K. H., Larsson, E., and Kõljalg, U. 2004. High phylogenetic diversity among corticioid homobasidiomycetes. Mycol. Res. 108: 983-1002.
LePage, B. A., Currah, R. S., Stockey, R. A. and Rothwell, G. W. 1997. Fossil ectomycorrhizae from the middle Eocene. Am. J. Bot. 84: 410-412.
Matheny, P. B. 2005. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Mol. Phylogenet. Evol. 35: 1-20.
Matheny, P. B. and Bougher, N. L. 2006. The new genus Auritella from Africa and Australia (Inocybaceae, Agaricales): molecular systematics, taxonomy and historical biogeography. Mycol. Progress 5: 2-17.
Matheny, P. B., Curtis, J. M., Hofstetter, V., Aime, M. C., Moncalvo, J. M., Ge, Z. W., Yang, Z. L., Slot, J. C., Ammirati, J. F., Baroni, T. J., Bougher, N. L., Hughes, K. W., Lodge, D. J., Kerrigan, R. W., Seidl, M. T., Aanen, D. K., DeNitis, M., Daniele, G. M., Desjardin, D. E., Kropp, B. R., Norvel, L. L., Parker, A., Vellinga, E. C., Vilgalys, R. and Hibbett, D. S. 2006. Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 982-995.
Matheny, P. B., Wang, Z., Binder, M., Curtis, J. M., Lim, Y. W., Nilsson, H. R., Hughes, K. W., Hofstetter, V., Ammirati, J. F., Schoch, C. L., Langer, E., Langer, G., McLaughlin, D. J., Wilson, A. W., Frøslev, T. G., Ge, Z. W., Yang, Z. L., Baroni, T. J., Fischer, M., Hosaka, K., Matsuura, K., Seidl, M. T., Vauras, J., Hibbett, D. S. 2007. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 43: in press.
Moncalvo, J. M., Lutzoni, F. M., Rehner, S. A., Johnson, J. and Vilgalys, R. 2000. Phylogenetic relationships of agaric fungi based n nuclear large subunit ribosomal DNA sequences. Syst. Biol. 49: 278-305.
Moncalvo, J. M., Vilgalys, R., Redhead, S. A., Johnson, J. E., James, T. Y., Aime, M. C., Hofstetter, V., Verduin, W., Larsson, E., Baroni, T. J., Thorn, R. G., Jacobsson, S., Clemençon, H. and Miller, O. K. 2002. One hundred and seventeen clades of euagarics. Molecular Phylogenet. Evol. 23: 357-400.
Pegler, D. N. and Young, T. W. K. 1969. Ultrastructure of basidiospores in Agaricales in relation to taxonomy and spore discharge. Trans. Br. Mycol. Soc. 52: 491-513.
Peintner, U., Bougher, N. L., Castellano, M. A., Moncalvo, J. M., Moser, M. M., Trappe, J. M. and Vilgalys, R. 2001. Multiple origins of sequestrate fungi related to Cortinarius (Cortinariaceae). Am. J. Bot. 88: 2168-2179.
Singer, R. 1986. The Agaricales in modern taxonomy. 4th edition. Koeltz Scientific Books, Koenigstein, Germany.
Taylor, T. N. and Osborn, J. M. 1996. The importance of fungi in shaping the paleoecosystem. Rev. Palaeobot. Palynology 90: 249-262.
Walther, G., Garnica, S., and Weiss, M. 2005. The systematic relevance of conidiogenesis modes in the gilled Agaricales. Mycol. Res. 109: 525-544.
Information on the Internet
- Assembling the Fungal Tree of Life. Source of multigene alignments and PCR primer information.
- Agaricales of the Hawaiian Islands. Treats diversity of Agaricales in Hawaii.
- Agaricales of Java and Bali. Treats diversity of Agaricales from southeast Asia.
- Fungimap. This website highlights some of the most conspicuous species of the mushroom flora in Australia.
- Macrofungi of Costa Rica. Diversity of mushroom-forming fungi from the neotropics.
- Molecular Systematics and Evolution of Agaricoid Mushrooms and Allies. Resource for ribosomal RNA gene sequence alignments and PCR primer information.
- Mykoweb: mushrooms, fungi, mycology. Devoted to the science of mycology and the hobby of mushrooming.
Title Illustrations

| Scientific Name | Gymnopilus spectabilis |
|---|---|
| Comments | agaricoid clade, Gymnopileae |
| Acknowledgements | Image used with permission |
| Specimen Condition | Live Specimen |
| Source Collection | MykoWeb |
| Copyright | © Mike Wood |
| Scientific Name | Lycoperdon perlatum |
|---|---|
| Comments | puffball; agaricoid clade, Agaricaceae |
| Acknowledgements | Image used with permission |
| Specimen Condition | Live Specimen |
| Source Collection | MykoWeb |
| Copyright |
© Taylor F. Lockwood

|
| Scientific Name | Squamanita paradoxa |
|---|---|
| Comments | Squamanita paradoxa, a mycoparasite of Cystoderma amianthinum. |
| Acknowledgements | Image courtesy of Juan Santos |
| Specimen Condition | Live Specimen |
| Copyright | © Juan Santos |
About This Page
Development of this page was facilitated by the "Assembling the Fungal Tree of Life" project (NSF award DEB-0228657)
We would like to thank Duur Aanen, Sigisfredo Garnica, David Grimaldi, David Hibbett, David LaPluma, Taylor Lockwood, Brooke Mahnken, Mykoweb, Juan Santos, Mark Steinmetz, and Greg Thorn for producing or sharing images posted on this page.
Brandon Matheny

Clark University, Worcester, Massachusetts, USA
Jean-Marc Moncalvo

Royal Ontario Museum and University of Toronto, Toronto, Ontario, Canada
Scott A. Redhead

Canadian National Mycological Herbarium, Agriculture & Agri-Food Canada
Correspondence regarding this page should be directed to Brandon Matheny at , Jean-Marc Moncalvo at , and Scott A. Redhead at
Page copyright © 2007 Brandon Matheny, Jean-Marc Moncalvo, and Scott A. Redhead
 Page: Tree of Life
Agaricales.
Authored by
Brandon Matheny, Jean-Marc Moncalvo, and Scott A. Redhead.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Agaricales.
Authored by
Brandon Matheny, Jean-Marc Moncalvo, and Scott A. Redhead.
The TEXT of this page is licensed under the
Creative Commons Attribution License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- First online 02 May 2007
- Content changed 09 May 2007
Citing this page:
Matheny, Brandon, Jean-Marc Moncalvo, and Scott A. Redhead. 2007. Agaricales. Version 09 May 2007. http://tolweb.org/Agaricales/20551/2007.05.09 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site